Calcium ions and healthy skin: Key process gives insight for future skin therapies
Our skin is the first line of defence from external harm and a central part of the thermoregulatory system. Dr Richard Evans and Dr Andrew Mayes of Unilever Research & Development in the UK, alongside a team of collaborators from the University of Liverpool, UK, present mounting evidence regarding the essential role of store-operated calcium entry (SOCE) for normal skin function. In a recent review article, members of the team describe the underlying mechanisms and chief proteins involved in detecting calcium levels and in regulating their influx and efflux in skin cells. Insight into these mechanisms gives scientists the power of knowledge to design treatments for associated skin conditions.
The primary function of our skin is to shield our tissues from the external environment. This organ, the largest in our body, protects us from physical harm and environmental threats such as ultraviolet (UV) light and dehydration. It also helps regulate our body temperature by sweating, which helps cool our skin. Emerging research suggests that a process known as ‘store-operated calcium entry (SOCE)’ underpins the normal physiological functioning of a range of skin cells.
SOCE is the process where stocks of calcium within cells become depleted, triggering the entry of calcium ions into the cell from the outside, and in turn, inducing the cells to respond in some way. In their latest review paper published in the journal Frontiers in Physiology, Dr Richard Evans of Unilever Research & Development and collaborators, Professor Caroline Dart and Dr Declan Manning at the University of Liverpool, UK, summarise the recent evidence for the vital role played by SOCE and the key proteins stromal interaction molecule 1 (STIM1) and Orai1 in the maintenance of healthy skin.
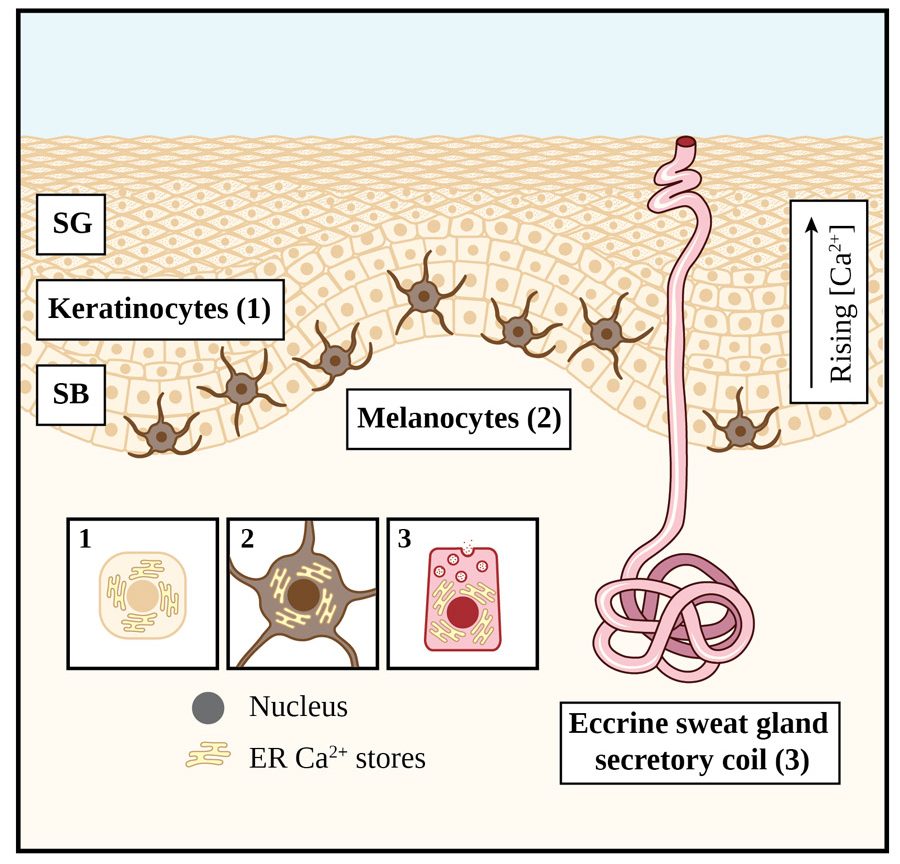
Emerging research suggests that a process known as ‘store-operated calcium entry (SOCE)’ underpins normal physiological functioning of skin cells.Three cell types which are prevalent in skin are keratinocytes, melanocytes, and eccrine sweat gland secretory coil cells (see Figure 1 for details on their location within the skin structure). The epidermis is the outer layer of the skin and consists mainly of keratinocytes. These cells need to differentiate in order to form the toughened or ‘cornified’ layer and provide protection from mechanical injury. Keratinocyte differentiation is also required for normal skin physiology and for maintaining healthy, hydrated skin.
SOCE: A critical factor in differentiation and skin condition
When keratinocytes in the basal or deeper layer of the epidermis proliferate or multiply in number, they are immature and unable to fulfil their function. To do so, they must go through the process of differentiation as they are ‘pushed up’ towards the outermost layer of the skin. In its simplest form, keratinocyte differentiation is the maturing of keratinocytes marked by changes in their characteristics, including shape and function. The process, however, is far more complex than that and involves a set of changes at the molecular and organelle level in cells.
Skin cell differentiation is driven by higher calcium concentrations in the outer layers, specifically the stratum granulosum, compared to the deeper layers (as shown in Figure 1). The calcium-sensing receptor (CaSR) detects this calcium gradient. It activates the messenger, inositol trisphosphate (IP3), which depletes calcium stocks and, in doing so, ultimately triggers SOCE (see Figure 2, which explains the process in each cell type mentioned) and cell differentiation by altering the expression (or level) of genes and proteins.

The importance of STIM proteins and Orai channels to SOCE and epidermal functioning has also been demonstrated by others in mice where reduction of STIM1 and Orai1 protein expression decreases keratin 1 expression, a protein essential for skin integrity. Furthermore, mice without Orai1 have thinner skin, emphasising the importance of Orai1 for normal skin structure and function.
If SOCE is dysfunctional, then calcium stores are not restocked. In such cases, this would impact the skin cells that help form the epidermal calcium gradient and drive keratinocyte differentiation. Such dysfunction could lead to dry or sensitive skin, and in extreme cases even more severe skin conditions such as dermatitis (skin irritation), anhidrotic ectodermal dysplasia (a genetic disorder affecting the skin, sweat glands, hair, and teeth), and hyperhidrosis (excessive sweating; see later) which are all known to be linked to abnormal SOCE and its proteins.

While Orai channels are thought to be the fundamental drivers of keratinocyte differentiation, evidence is emerging that they are not the only channels regulating calcium influx in these cells. It is now becoming increasingly evident that transient receptor potential canonical (TRPC) proteins are likely to play a role in SOCE. In their latest work, also published in Frontiers in Physiology, Manning, Dart and Evans use high-resolution microscopy of keratinocytes to explore the potential mechanisms underlying TRPC1 channel activation.
Melanin and skin pigmentation
When exposed to sunlight, melanocytes produce the skin pigment melanin to protect the keratinocytes from damage which could be induced by the ultraviolet (UV) radiation in sunlight. Melanin production leads to darkening or ‘tanning’ of the skin. The processes and functions of melanocytes are known to be regulated by melanocyte-inducing transcription factor (MITF), which controls a number of associated genes. Upregulation of MITF causes skin pigmentation and can lead to either adaptive melanogenesis (or ‘adaptive tanning’), or to standard baseline melanogenesis.
A deeper understanding of SOCE as a fundamental process underlying normal physiological function of skin opens avenues for further research and therapy development.Alpha-Melanocyte Stimulation Hormone (α-MSH) activates the melanocortin one receptor (MC1R) in a well-established pathway central to melanin production. In fact, mutations in MC1R genes can be found in fair-skinned, red-haired people with low melanin levels. Recent findings point towards a role played by calcium-dependent proteins such as STIM1 in this pathway, with experiments demonstrating that reducing STIM1 decreases melanin production.
Other studies suggest that SOCE and Orai1 have a part to play in adaptive melanogenesis, building evidence for the role of SOCE in regulating the process of skin darkening and its final colour. The role of SOCE may be particularly relevant to body areas prone to increases in skin pigmentation. These areas include the underarm (or axilla) where irritation from hair shaving or plucking, and the use of harsh soaps and ‘enthusiastic’ towel drying can lead to some skin darkening over time.
Hot and sweaty: SOCE in thermoregulation
The eccrine gland is the ‘sweat gland’ that produces sweat to cool the skin and the body. This process begins with the neurotransmitter acetylcholine stimulating the release of calcium from intracellular stores, triggering calcium influx from external sources by SOCE (see Figure 2) in the secretory coil cells of the eccrine gland. The second step in the process of sweating involves the movement of chloride ions across the secretory coil cells into the glandular lumen. Cultured cell and human studies again implicate STIM1 and Orai1 as the key proteins in the SOCE process required for sweat secretion. The strongest evidence for their role in the process is that deleting expression of these proteins (by gene knockdown) in model systems results in impairment of the calcium signal, and by implication sweating. Conversely, eccrine coil cells from people with hyperhidrosis (excessive sweating) reveal overexpression of STIM1, an exaggerated calcium influx and SOCE response, and consequently enhanced sweating.
A deeper understanding of SOCE as a fundamental process underlying normal physiological function of the skin opens avenues for both further research and therapy development. Evans and collaborators have summarised existing data and provided new insights in their recent publications which emphasise just how pivotal the role of calcium regulation and SOCE is for skin health. They have also identified gaps in our knowledge, highlighting focus areas for future work. Considering SOCE’s significant role across a range of skin functions, improved understanding of the underlying mechanisms will certainly provide opportunities for the development of better treatments for a diverse range of skin disorders.

Personal Response
What inspired you to conduct this research?Since calcium ions and the process of store-operated calcium entry (SOCE) are central to the healthy functioning of many cell types, we were keen to understand their role in skin. We were amazed to see how pivotal SOCE, and key proteins STIM and Orai (named after the ‘keeper of heaven’s gate’ in Greek mythology due to their vital role) were in determining the condition of human skin, its colour, and indeed the sweating response that enables humans to temperature-regulate. This inspired us to undertake our work on the role of TRPC1 in keratinocytes, and additional future studies.
How might a better understanding of the SOCE mechanism help in preventing skin conditions?
Understanding SOCE and the roles of different STIM and Orai isoforms in controlling key processes such as keratinocyte differentiation, melanogenesis, and sweat production will provide deep insights into the consumer-relevant outcomes of skin condition, skin colour, and sweating. It may be possible to generate cosmetic solutions which provide beneficial changes to these outcomes, or the insights that help design specific solutions to conditions which may be classified as being more severe.