Self-assembled nanomaterials fight viral outbreaks
Developing efficient ways to treat viral infections and control their spread in humans and animals has become a worldwide priority, especially since the COVID-19 pandemic. Traditional approaches to creating vaccines suffer from very long development and testing times which make them unsuitable for treating new viral infections. Dr Alaa F Nahhas at King Abdulaziz University, Kingdom of Saudi Arabia, shows how technologies based on the use of self-assembled nanoparticle materials provide a powerful novel route to treating existing and future viral outbreaks.
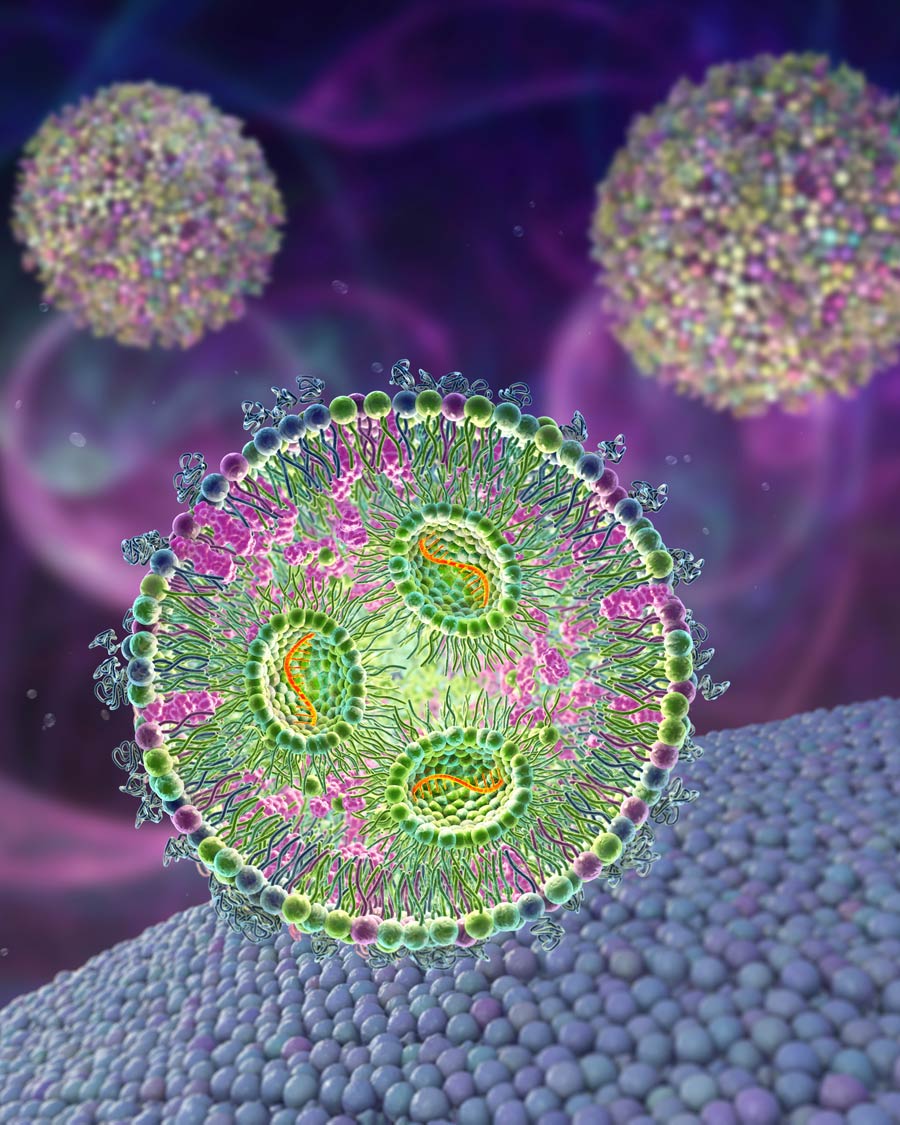
Viruses are sub-microscopic pathogens that replicate inside the cells of living organisms. They are composed of genetic material, either DNA or RNA, surrounded by a protein coat called a capsid. Some viruses may also have an outer envelope composed of lipid molecules. Viruses lack the ability to reproduce or carry out metabolic processes on their own. Instead, they rely on host cells to provide the necessary machinery for their replication.
Viruses can infect a wide variety of organisms, including animals, plants, and even bacteria. They are responsible for causing numerous diseases in humans, such as the common cold, influenza, HIV, hepatitis, smallpox, measles, herpes, Middle East respiratory syndrome (MERS), and COVID-19, among others. When a virus infects a host cell, it hijacks the cellular machinery that keeps the cell alive and forces it to produce more viral particles. These particles can then infect other cells, spreading the infection throughout the organism.

Viral infections are typically very difficult to treat and historically, major viral outbreaks have always posed severe healthcare problems, both in advanced societies and in developing countries. Vaccines are available for most known viral diseases. However, the time required to develop and test these vaccines has proved to be, in many cases, prohibitively long. For instance, it took five years to develop a vaccine for measles, 46 years to develop the poliovirus vaccine, and over a millennium to discover a vaccine for smallpox.
As new viral strains continuously appear, our inability to develop and deliver suitable vaccines in short times can lead to uncontrollable viral spread.As new viral strains continuously appear, our inability to develop and deliver suitable vaccines in a short period of time can lead to uncontrollable viral spread, potentially on a global scale, as in the case of COVID-19. Building on expertise accumulated during the COVID-19 pandemic, Dr Alaa F Nahhas at King Abdulaziz University, Kingdom of Saudi Arabia, proposes a set of innovative approaches to quickly and efficiently develop means to contain and treat new viral outbreaks.
Self-assembled nanoparticles
Nahhas’s research is devoted to the application of nanomaterials to target and kill viruses without causing undesired side effects in humans. A nanomaterial is a material whose fundamental units, or nanoparticles, have sizes of the order of 1–100 nanometres, where 1 nanometre corresponds to a billionth of a metre. Nanomaterials find widespread application in various therapies including cancer treatment, neurological diseases, and infectious diseases. Examples include liposomes – nanoparticles extensively used for drug delivery. They spontaneously assemble to form lipid-like structures in water, creating spherical vesicles that efficiently capture hydrophilic drugs, thereby improving drug stability and prolonging their effectiveness. Dendrimers, an important example of polymeric nanoparticles, can be composed of synthetic or natural building blocks, like amino acids. Their size is determined by surface functionalisation, and they possess a central cavity capable of loading small drug molecules and an outer layer that can be chemically modified to target specific attachment sites on the surface of a virus. Finally, virus-like particles (VLPs) are self-assembled particles composed of several viral proteins. When genetic material is incorporated in VLPs, these nanoparticles can stimulate an immune response, effectively acting as a vaccine.
Peptide nanomaterials
Nahhas’s work has focused on the use of peptides as potential candidate drugs for disrupting the lifecycle of viruses. Peptides are short sequences of chemically bound amino acids which self-assemble to adopt characteristic three-dimensional structures with specific physiological functions. Insulin, a hormone used in the treatment of diabetes, is a well-known example of a therapeutical peptide. Peptides can penetrate cell membranes, attach to the surface of a virus, or even enter its lipid envelope and act as vehicles for drug delivery. They exhibit low toxicity in humans and higher specificity than conventional drugs. In practice, however, they can be relatively easily degraded and inactivated by specific enzymes such as proteases, and they may therefore exhibit short lifetimes in physiological conditions.
Therapeutical approaches based on nanomaterials are growing into a powerful new technology for containing and treating future viral outbreaks.One potential way to overcome the limitations of peptides as drug delivery agents is the use of suitable peptide carriers, such as nanoparticles like micelles, liposomes, and polymeric particles, which protect the therapeutically active peptide from enzymes. Another approach consists of chemically modifying a peptide to make it less sensitive to degradation. Nahhas has studied how the incorporation of amino acids not normally found in natural proteins, enzymes, and peptides can increase the resilience of therapeutically active peptides in physiological conditions.
Peptide hydrogels
Nahhas has examined how the properties of self-assembled peptide hydrogels, which are crosslinked polymeric networks of amino acids capable of holding large amounts of water and potentially suitable as drug delivery agents, are modified through the incorporation of D-amino acids. D-amino acids have chemical structures very similar to their natural counterparts (L-amino acids) from which they differ only in their chirality, that is their ability to rotate polarised light in different directions. Crucially, D-amino acids enhance the stability of a peptide to protease degradation. Nahhas’s results have shown that D-amino acid modified hydrogels and hydrogels containing α-aminoisobutyric acid, another amino acid not observed in natural proteins, exhibit remarkable activity as antimicrobial and anti-cancer agents.
Nanomaterials fight COVID-19
Nahhas’s expertise in engineering nanomaterials for therapeutical use proved to have far-reaching consequences during the COVID-19 pandemic. She proposed modifying self-assembling nanomaterials previously developed for application in tissue engineering and using them to reduce the ability of the SARS-CoV-2 virus to spread in mammalian tissues. The idea was that suitably formulated nanomaterials could self-assemble around the virus, ‘blanketing’ it and preventing it from entering new cells to start it replication. The key point of this strategy was to develop an understanding of how a nanomaterial could be functionalised to increase its affinity for the virus.
Nahhas used information from the amino acid sequence of the protein enveloping SARS-CoV-2 to synthesis peptides with a structure complementing that of the enveloping protein. She used extensive molecular dynamics simulations, a computational technique that allows one to study in real time the conformation and dynamics of complex molecules, to propose a self-assembling nanomaterial that could inhibit SARS-CoV-2 replication. This molecule was then commercially released, and extensive in vitro and in vivo studies were carried out to assess its ability to passivate SARS-CoV-2. Computational modelling was the key to achieve this goal quickly and in a time in which most experimental facilities were unavailable owing to safety restrictions. ‘We would not have developed a therapy or so many other technologies to help fight COVID’, says Nahhas, ‘had it not been for computational nanomedicine.’
Facing future viral outbreaks
The lessons learned during the COVID-19 pandemic and the continuous improvements in the efficiency and speed of protocols for the creation of new nanomaterials for therapy are providing a powerful set of tools to detect, study, and control viruses responsible for major lethal outbreaks, from COVID-19 variants, to Ebola, to the Marburg virus. Similar methods can also be used to control common viral infections, like the respiratory syncytial virus (RSV), a usually relatively mild condition that can lead to serious complications in children and older adults.
New concepts are being developed thanks to the synergy between experimental work and computational modelling. Establishing robust safety practices to handle dangerous viruses in the laboratory and prevent uncontrolled spread is also a priority in view of the appearance of new strands of existing viruses or of completely new viral species. Molecular imprinting, which creates self-assembled polymeric matrices with high affinity for specific substrates, provides a convenient way to avoid using real viruses for research and minimises chances of potentially powerful pathogens being released in the atmosphere. ‘Therapeutical approaches based on nanomaterials’, says Nahhas, ‘are transforming our ability to face new challenges, and they are growing into a powerful new technology for containing and treating future viral outbreaks.’
Personal Response
What are the advantages of nanomaterial-based medicine compared to traditional vaccine development methods?Using nanotechnology in the medical field has many advantages as a drug delivery system over other traditional drugs. Water-soluble drugs can be difficult to absorb on the targeted site; however, using nanotechnology can increase the absorbability of these drugs. Also, a problem with many drugs is that they are cleared from the body too quickly before doing their function. Nanotechnology can solve this problem by increasing the time period of these drugs in our bodies. Nanomedicine is also used to reduce drug volume, especially that affecting healthy tissue, because the main consideration in designing cancer drugs is to design a drug that targets just cancer cells, not normal healthy cells.