Atmospheric species and their effect on solar radiation and our health
Our atmosphere is a giant chemical reactor that helps keep us safe from the potentially damaging and warming effects of solar radiation. It consists of a hugely complex mixture of atmospheric liquids, gases, and particles with constantly varying compositions. Professor Jianhui Bai at the Chinese Academy of Sciences and Professor Rui Chen at the Beijing Academy of Science and Technology have been studying how some of the particulate matter affects the chemical reactions that take place in the atmosphere and their interactions with solar radiation, and how ultrafine particles may also affect our health.
The Earth’s atmosphere plays many roles in keeping the Earth a habitable and safe place for its human population. As well as providing the oxygen we breathe, the atmosphere also protects us from the full intensity of the sun’s radiation. This protection is in the form of absorption, reflection, and attenuation of nearly all shorter-wavelength radiation from the sun. The depletion of the ozone layer over Antarctica allows much more UV radiation to pass through the atmosphere, leading to a disruption of global temperatures and environment.
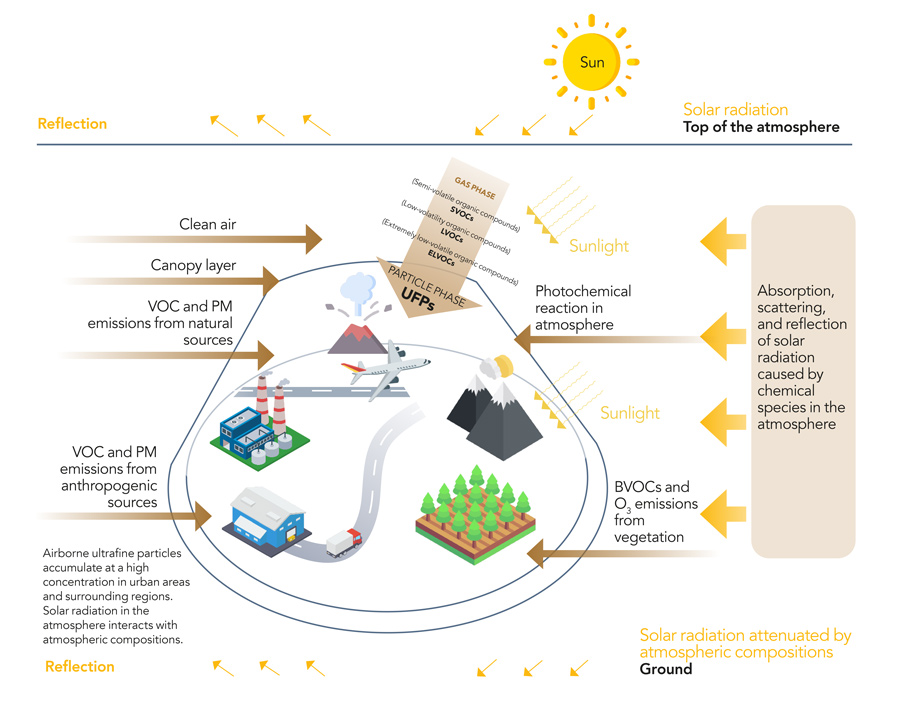
What happens in the atmosphere with solar radiation is complex. Some of the radiation is reflected or scattered back away from the Earth. Some is absorbed by the myriad of chemical species in the atmosphere, triggering light-initiated chemical reactions through hydroxyl radicals (OH), excited nitrogen dioxide (NO2), water vapour, and volatile organic compounds (VOCs) in the short-wavelength region (UV and visible). When these so-called photochemical reactions start to occur, they can trigger a whole cascade of further reactions that ultimately end up changing the chemical composition of the atmosphere, as well as the distribution of solar radiation in the atmosphere and at the Earth’s surface.
A threat to human health
Most chemical species in the atmosphere are either in the form of gases or vapours. Another species that makes up a large amount of the atmosphere is particulate matter. Particulate matter are microscopic solids or liquids that are generally categorised according to the size of the particle.
Atmospheric particulate matter has been of great interest recently as there have been growing concerns that it can pose a threat to human health. A large amount of particulate matter in our atmosphere is produced from human sources, such as wood burning and the breakdown of tyres and car brakes. Often, air quality is measured by monitoring the concentrations of particulate matter of certain sizes, with high concentrations of some of the smallest particulate species (like PM2.5) being the most concerning. Ultrafine particulate matter is not just produced by anthropogenic, or human, sources – some is made in the atmosphere naturally as a result of the many photochemical reactions that take place.
There have been growing concerns that particulate matter can pose a threat to human health.While the importance of the atmosphere in regulating Earth’s climate, water and weather systems is clear, what is not as clear is how to understand the ever-changing chemical soup that makes up the atmosphere. With such an interconnected network of dependencies in terms of chemical and photochemical reactions, working out the role of an individual species can be challenging.
Professor Jianhui Bai at the Chinese Academy of Sciences and Professor Rui Chen at the Beijing Academy of Science and Technology are undaunted by the challenge of trying to understand the relationship between solar radiation and atmospheric composition and how each individual chemical species either absorbs or scatters light. By performing long-term studies to monitor how solar radiation levels change and their relationship to the weather at the time, they are building up models and an understanding of just how long-term variations might influence our overall climate.
Climate measurements
One of the challenges for scientists like Bai and Chen is how to make meaningful measurements on a system as big as the Earth. It is not possible to monitor all regions of the globe and atmosphere simultaneously, so sometimes specific monitoring sites must be selected.

One such site that the team have been studying data from since 2007 is Qomolangma, a region better known in the Western world as Mount Everest. Mount Qomolangma in Tibet is one of the highest regions in the world and an ideal place for atmospheric scientists to make measurements to study the atmospheric composition without the need for aeroplanes.
Since 2007, the team have been monitoring the solar radiation levels and weather conditions using the Qomolangma Atmospheric and Environmental Observation and Research Station, set up by the Chinese Academy of Sciences. They would record hourly data which was then used to develop a mathematical model to predict the amount of solar radiation and which factors in the atmosphere led to changes in the amount of solar exposure.
The research team were able to successfully create a model that could describe historical variations in the solar energy levels very well and found a huge number of seasonal and daily patterns and trends in the amount of radiation exposure. The team were able to identify a number of chemical species that were responsible for the different transformation processes of solar radiation, including absorption, scattering and reflection, and discovered that the influences of the concentrations of certain species on the overall radiation exposure was highly non-linear.
They also found a continual rise in average temperatures and discovered that the total amount of solar radiation losses caused by the gaseous, liquid, and particulate matter in the atmosphere seemed to be decreasing by a few percent, whereas the absorbing loss increased. The biggest losses though were in the amount of absorbing and scattering chemical species that indicate that these gaseous, liquid, and particulate species have an important role to play in controlling local heating.
Ultimately, from establishing a clear link between the concentrations of atmospheric species and the amount of solar radiation exposure, Bai and Chen concluded that particular care needs to be taken to limit the number of atmospheric species that absorb solar radiation. It is these chemical species (all gases, liquids, and particles, including greenhouse gases, or GHGs, and non-GHGs) that seem to contribute the most to climate change/warming in the regions studied. Therefore, their emissions and production through chemical and photochemical reactions is of most concern, and the researchers suggest this needs to be reduced to slow climate warming.
It is these chemical species that seem to contribute the most to climate warming in the regions studied.Health effects
It is not just the climate and atmospheric composition that are of concern from the changing concentrations of particular chemical species. Bai and Chen and their teams have been looking at how particulate matter not just changes the atmospheric composition but also affects human health.
Combustion processes and the burning of fuels and biomass release many chemical species into the atmosphere that can lead to the greater production of chemical species such as ozone as well as BVOCs (biogenic volatile organic compounds). BVOC oxidation is an important source of small-sized aerosols. Combustion also produces a large number of small aerosols and particulates that recent research shows can accumulate in the human body.
When we breathe in the air around us, we also breathe in small particulate matter that is too small to be filtered out by the structures in our nose designed to prevent any foreign bodies from entering. Ultrafine particulate matter can even reach our brain tissue, where it can accumulate.
Right now, it is unclear exactly what safe exposure levels are for different kinds of particulate matter and how they link to different diseases. There are fears that uptake of these species may be related to neurodegenerative disorders.
The research team have been trying to understand how ultrafine particulate matter differs in terms of its potential health impacts versus more commonly monitored larger particulate species like PM2.5 and whether ultrafine particulate matter really does have unique nanotoxicity behaviour.
At present, it is clear that ultrafine particulate matter can be inhaled and has the potential to pose a risk but there is more research required to properly understand whether it is truly toxic. Larger particulates are known to cause irritation to the eyes, lungs, and throat and can even lead to corrosion of the lungs due to the particle’s ability to embed itself into the tissues there. It may turn out that minimising ultrafine particulate production is not just what is best for our environment, but best for our health as well.
Personal Response
What inspired you to conduct this research?Climate change is a big challenge. The polar regions are the most sensitive regions to climate warming. Solar radiation drives the atmosphere and interactions between the atmosphere, biosphere and land, and controls the regional energy balance in the atmosphere and at the surface. Atmospheric compositions with different absorbing and scattering properties are regional dependent, resulting in the redistributions of solar radiation and atmospheric compositions. It is an important task to fully investigate the multiple interactions and their mechanisms between solar radiation, atmospheric constituents, and the climate.
How concerned should we be about ultrafine particulate matter?
There is no need to panic. Humans are inevitably exposed to a wide range of fine and ultrafine particles in the environment. Ultrafine particles have received increased attention due to their unique properties. What we should be concerned about is avoiding long-term exposure to the anthropogenic-source particles as much as possible.