Glycomimetic peptides as powerful anti-inflammatory treatments
Glycan (sugar)-binding lectin-type receptors on immune cells have the potential to serve as therapeutic targets. However, many of these receptors are not targeted because of a lack of suitable binding agents. Professor J Kenneth Hoober and Dr Laura L Eggink, co-founders of Susavion Biosciences, Inc, and Wild Boar Biosciences, LLC, have created peptide mimetics of glycans that show greater flexibility compared to rigid glycans and thus stronger binding to receptors – characteristics that open them up as potentially powerful anti-inflammatory drugs.
Glycobiology is the study of the structure and function of glycans, also called carbohydrates or sugar chains. Glycans coat the surfaces of all cells, are attached to most serum proteins and essential for the function of living organisms. The position of glycans on the cell surface makes them well-suited for interactions with surrounding cells. These interactions are enabled by lectins – proteins that bind to specific glycan molecules. Immune cells express numerous lectin-type receptors, and glycan–lectin interactions play a major role in how the immune system works.
Lectin-type receptors therefore offer a potential repertoire of novel targets for drug development. However, many of these receptors have not been exploited as therapeutic targets due to lack of suitable ligands (molecules that bind to receptors).
The discovery of peptides that mimic the natural glycan ligand of these receptors offers a new approach to modulate the immune system.
Glycans vs glycomimetic peptides as potential treatments
For ligands to be active as drugs and stimulate an immune response, they must bind to receptors strongly, that is, with ‘high affinity’. Affinity is a measure of the overall strength or stability of multiple bonds occurring simultaneously. Glycans have rigid structures and their flexibility is limited to the glycosidic bonds between the individual sugar molecules.
Therefore, affinity depends on the extent to which the structure of the ligand and the shape of the protein surface within the binding site fit together. Glycans generally bind with low affinity, but multivalent glycan compounds (that is, where multiple glycans are bound together) have improved affinity. Another approach to achieve higher affinity of glycans has been to attach peptides that bind a receptor adjacent to the glycan binding site. Nevertheless, these compounds often have poor drug-like characteristics.
The discovery of peptides that mimic glycans as ligands of these receptors offers a new approach to modulate the immune system.Dr J Kenneth Hoober and Dr Laura L Eggink of Wild Boar Biosciences LLC have been investigating the potential therapeutic use of glycomimetic peptides as ligands of lectin-type receptors expressed by cells of the immune system. Glycomimetic peptides (peptides that mimic the structure and function of glycans) have a much higher affinity for a binding site than glycans. Apart from the planar peptide bond, peptides have a flexible structure, with many conformational degrees of freedom, allowing them to adapt to the shape of the binding site by a mechanism known as ‘induced fit’. In an induced-fit type of binding, both receptor and ligand select the most stable (lowest energy) arrangement. Its high flexibility enables a peptide ligand to achieve optimal contact and the most favourable energy level in the binding site. Also, a small mimetic peptide can bind in the site with no additional components. Of note, in their multivalent form, peptides have higher overall affinity than in their monovalent (single molecule) form as the result of the ligand density or cluster effect (increased local concentration). These features of glycomimetic peptides makes them suitable as potential drugs.
Novel glycomimetic peptides for investigation
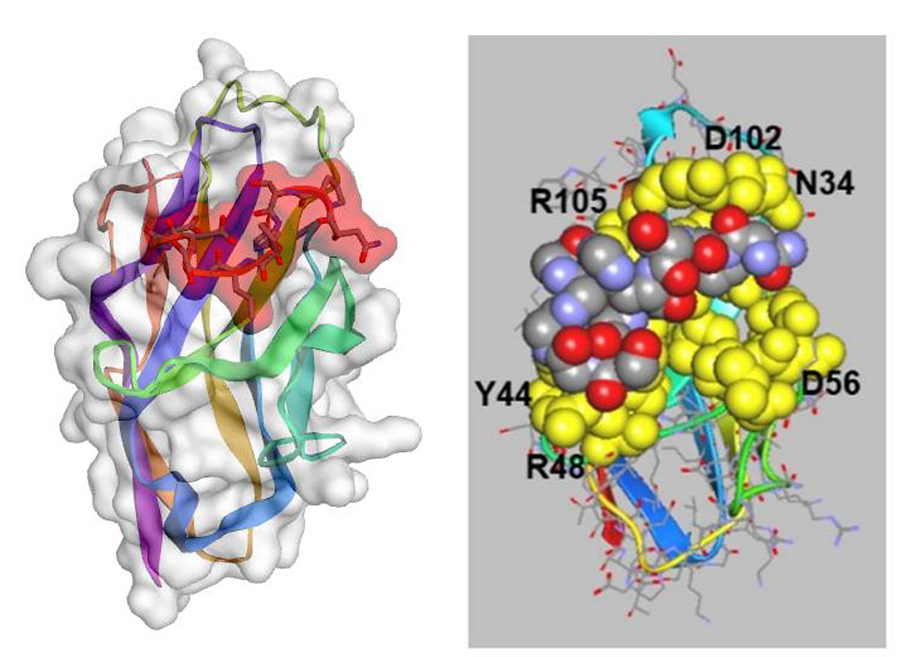
Many glycomimetic peptides have been identified by using lectins to screen phage display libraries. Hoober and Eggink identified a potential peptide from screens of a phage display library with plant lectins. Using in silico (computer-based) modelling with crystal structures of receptors from the literature and direct in vitro binding assays with sugar-specific lectins, they modified the selected peptide to create one with the sequence NPSHPLSG (named svH1C), which bound to lectins specific for sialic acid (Figures 1–3).
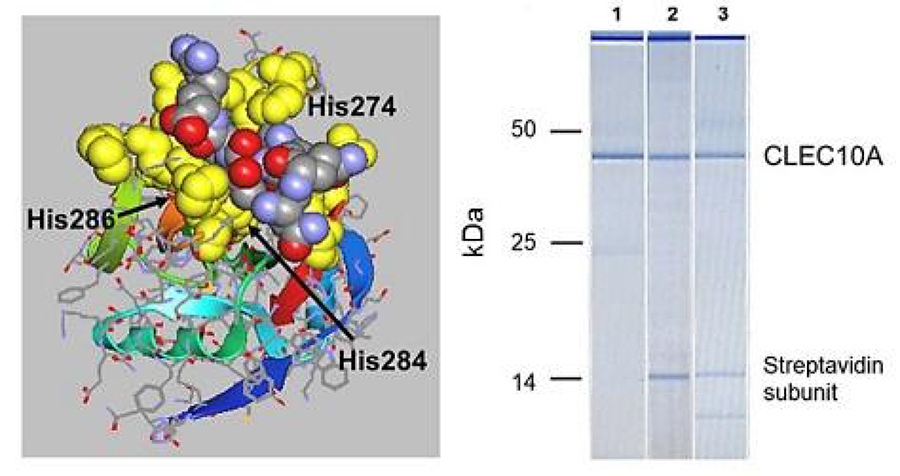
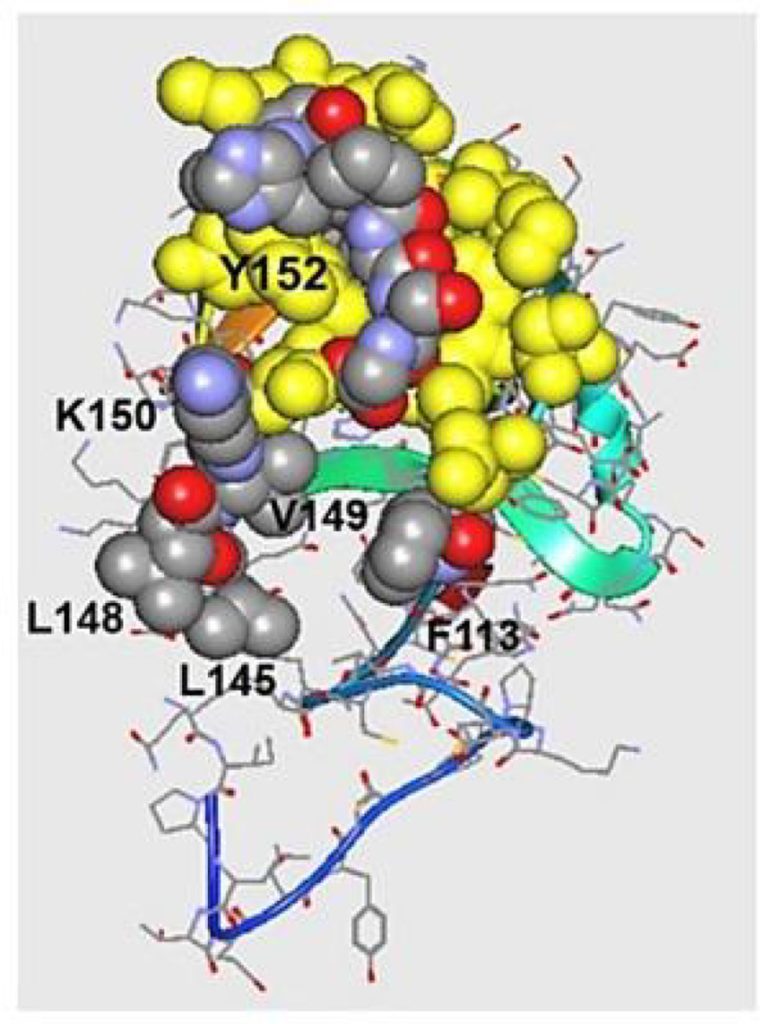
Hoober and Eggink also found a second peptide, with the sequence VQATQSNQHTPR, through screening of a phage display library with the N-acetylgalactosamine (GalNAc)-specific snail lectin. They synthesised a tetravalent structure of the peptide (designated svL4), which binds to GalNAc-specific human receptors ASGR1 and CLEC10A. The researchers synthesised the two halves of VQATQSNQHTPR separately, because its long structure extends beyond the sugar binding site. The resulting shorter peptide, NQHTPR, contained the GalNAc-binding activity (Fig 4, lane 2), and was synthesised as a tetravalent peptide (known as sv6D).
Hoober and Eggink extensively used an in silico programme to study peptide binding. The programme was vetted by comparing modelling results with in vitro experimental binding data. One example was a peptide that interacts with a glycan-binding site with very high affinity. Tendamistat is a naturally occurring, small protein inhibitor of the enzyme α-amylase. Crystallographic data showed that tendamistat forms a loop structure that binds into the catalytic (active) site of α-amylase with very high affinity. Using in silico modelling, Hoober and Eggink found that a 6-amino acid peptide with the amino acids that formed the loop bound to the crystal structure of α-amylase with nearly the same affinity as intact tendamistat. However, binding of flexible peptides results in a negative change in entropy, which with the intact tendamistat structure is offset by the restriction of movement on the loop sequence.
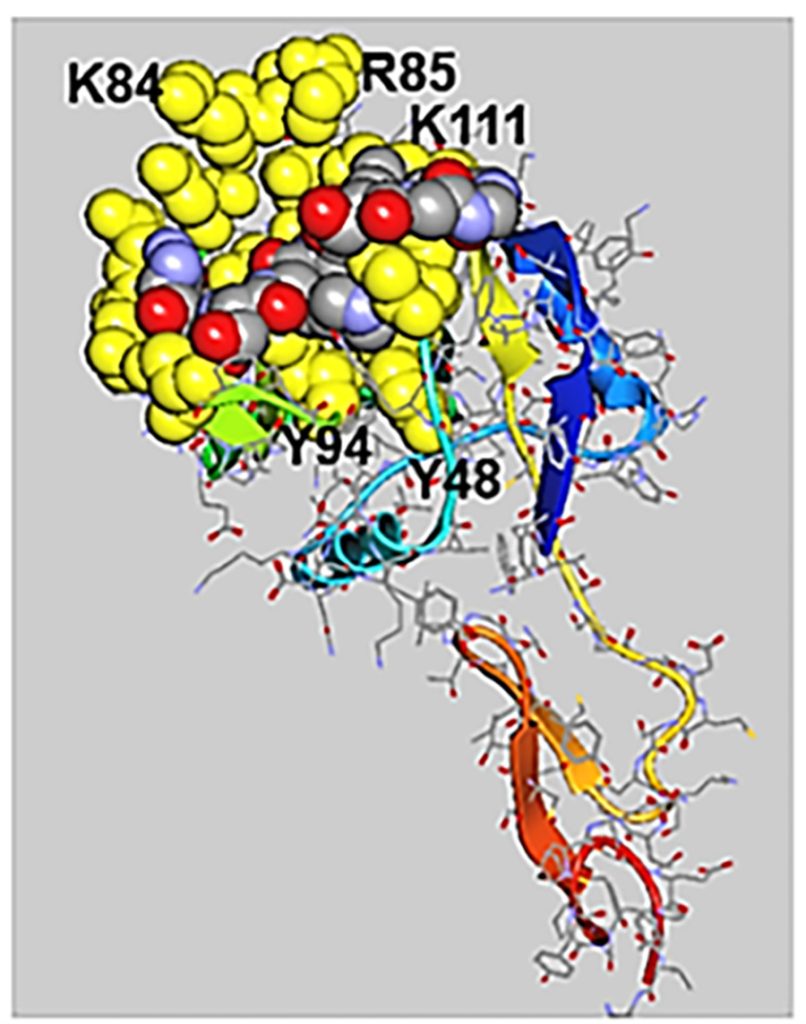
A second example compared binding of the peptide svH1C to NKG2D, an activating receptor found mainly on cells of the cytotoxic arm of the immune system. NKG2D is a key molecule in the activation and regulation of natural killer (NK) cells (a type of cytotoxic lymphocyte). In silico modelling of binding of svH1C to NKG2D provided a similar predicted binding energy (ΔG) as an analysis by microcalorimetry. The glycoprotein fetuin (a liver-made protein) inhibited the binding of svH1C to NKG2D to the same extent as it inhibited the binding of svH1C to the sialic acid-specific receptor Siglec-1, which suggests that the peptide binds to these receptors with approximately the same affinity.
Then, using both in silico and mouse models, they tested peptide mimetics of the glycans sialic acid and GalNAc.
Peptide mimetics of sialic acid and their receptors
Most glycoproteins in the human body have sialic acid-containing glycans that bind to lectin-type, cell-surface receptors. Hoober and Eggink focused their investigations on three of these receptor types. The first is the family of lectin-type receptors known as sialic acid-binding immunoglobulin-like lectins (siglecs), which are found on cells of the human immune system. The second is NKG2D, a receptor on natural killer cells (mentioned above). The third are the P- and E-selectins, C-type (Ca2+-dependent) lectin receptors that play a critical role in inflammatory diseases and are expressed by endothelial cells.
Anti-inflammatory potential of svH1C
Hoober and Eggink tested the idea that several critical, sialic acid-binding receptors can be engaged by a single peptide, svH1C, to enhance an anti-inflammatory therapy. They proposed that the flexibility of the peptide would allow the ligand to adapt to fit the shape of several receptors that bind the same glycan.
Most siglecs expressed by immune cells act as checkpoint inhibitors. When peripheral blood mononuclear cells (PBMC) were incubated with svH1C, dephosphorylation (inactivation) of the inhibitory siglecs occurred. Several siglecs lack a cytoplasmic regulatory domain and function in association with an activating protein. svH1C activated phagocytosis of antibody-coated particles by macrophages (Fig 5), which led to the complete inhibition of replication of HIV-1 in PBMC cultures, which suggests that svH1C could be useful as an anti-viral drug.
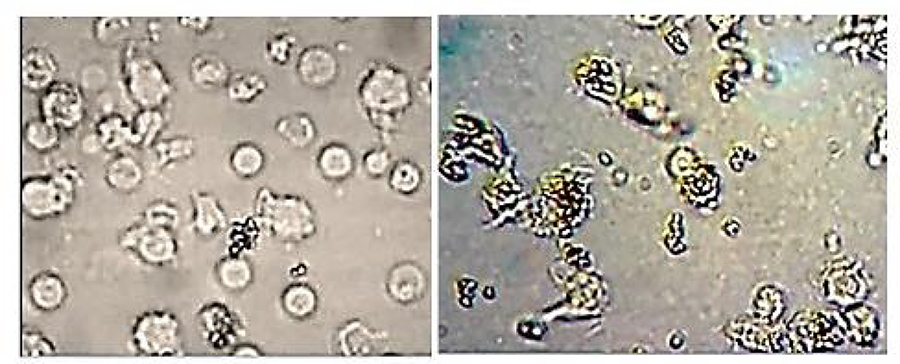
In silico models also indicated that P-, E- and L-selectin bound svH1C in the site of the natural glycan ligand, the sialyl Lewisx glycan (sLex), in the crystal structures of the receptors. Additionally, svH1C appeared to have similar affinity to each of the receptors. In mouse studies, subcutaneous injections with svH1C reversed inflammation caused by topical application of the bacterial endotoxin lipopolysaccharide and completely restored healthy skin. In addition, the neutrophils were cleared from the skin, which was an indication that migration of the inflammatory neutrophils from the blood into the skin was blocked by the peptide (Fig 6).
Peptide mimetics of N-acetylgalactosamine (GalNAc) and their receptors
Hoober and Eggink investigated the GalNAc-specific receptor CLEC10A (C-type lectin domain family 10 member A, CD301), a human C-type receptor found mainly on M2a macrophages and dendritic cells. The researchers showed by in vitro experiments with human dendritic cells that sv6D bound with high specificity to CLEC10A (Fig 4). In silico modelling revealed that sv6D bound to the receptor in the site identified by crystallography as the binding site of GalNAc. sv6D activated phagocytosis of opsonized microspheres by monocytes and macrophages from peripheral blood mononuclear cells. Opsonization is a process by which antibodies tag substances for phagocytosis, clearance by the immune cells. Their studies have shown that CLEC10A is both agonistic and antagonistic based on extent of occupancy of the receptor.
Outcomes from studies in mice implanted with ovarian cancer cells and injected with sv6D and svL4 indicated that dendritic cells were activated resulting in an enhanced anti-tumour state. Similarly, survival of mice in which a glioma cell line was implanted in the brain was dramatically extended by injections of svL4. Tumour growth was strongly inhibited and a several-fold increase in circulating peripheral blood monocytes and macrophages was found in and around the tumour.

Likewise, in mice with induced neutrophilic-driven eczema, topical svL4 completely repaired the epidermis within 14 days and cleared neutrophils from the dermis. Hoober and Eggink concluded that svL4 and sv6D provided a substrate for transglutaminase-2, which catalysed cross-linking of the surface of the skin, in addition to their ability to stimulate phagocytic activity in the dermis. These results suggest a potential two-pronged approach for treatment of skin inflammation.
A promising strategy for immune therapy
These data supported the hypothesis that despite the differences in the shape of the binding sites, the flexibility of the peptides allows them to bind to each of the tested sialic acid- and GalNAc-specific receptors with enough affinity to provide efficacy as drugs.
The peptide mimetics demonstrated much greater affinity for the receptors than glycans, and, therefore, have potential to engage lectin-type receptors on immune cells as therapeutic tools.
In silico modelling used in conjunction with confirmatory direct binding assays can help to advance understanding of ligand binding to lectin-type receptors.
Personal Response
What is next for your research?Studies should continue on the binding of svH1C to selectins. The peptide should also be tested in an experimental system in which the effect of the peptide on migration of neutrophils through endothelial tissues can be determined. The accumulated data should then be presented to the regulatory authorities for permission to conduct clinical trials.
What developments do you anticipate will happen in the next few years with respect to glycomimetic peptides research as potential therapies for immune diseases?
Inflammation is a major cause of disease. Efforts have been made for decades to find a druggable molecule to inhibit migration of neutrophils in order to reduce inflammation in peripheral tissues. We have found a peptide that achieves this goal and should be applicable to
treatment of most types of inflammation. Acceptance of peptides as drugs will continue to increase as more are found to be powerful therapeutic agents.
What do you see are the main obstacles to the research or use of glycomimetic peptides as potential therapies?
The obstacles are primarily two-fold: first, a greater acceptance of the efficacy and value of peptides as mimetics of carbohydrates and their potential as therapeutic drugs. Second, funding has been limited because of the perception of the risk involved (as indicated in the first obstacle).