Ketamine: An old medication with newly discovered actions in pain and breathing regulation
Pain that lasts longer than the expected period of healing is tormenting a significant percentage of the population and is the leading cause of disability worldwide. Although medications are available to control it, its management often involves trial and error, especially in cases where specific nerve damage cannot be found. Professor Albert Dahan at the Leiden University Medical Center in the Netherlands is studying a controversial and often overlooked drug called ketamine, including its role in pain management and mood disorders, but also in regulating heart function and breathing.
Chronic pain is defined as pain that lasts longer than three months or longer than the expected healing time after an injury or surgery. However, chronic pain is not just pain secondary to an injury that hasn’t yet healed; the mechanisms behind it are much more complex. It occurs when the nervous system responds in unusual ways that don’t accurately reflect what is going on inside the body. This can be explained by a persistent state of reactivity of the nervous system, even after the initial area of damage heals. In a vicious circle, pain causes the nervous system to be more alert (excited), and the alertness itself leads to more sensitivity to various stimuli and pain. Chronic pain is difficult to treat and affects the individual’s quality of life, often leading to severe disability.
Challenging cases of pain
There are different types of chronic pain that are challenging for clinicians to manage, including Complex Regional Pain Syndrome Type 1 (CRPS-1). CRPS-1 often affects one or more limbs after injury or surgery to the area, but it can also occur spontaneously. The mechanisms behind this syndrome are not clear yet, especially since there is no specific nerve damage found to explain the symptoms. The management of CRPS-1 is similar to that of any other chronic pain and often requires trying different treatments to find out what works best for each individual.
Another widespread type of chronic pain that poses a challenge to clinicians is neuropathic pain. This appears secondary to nerve disease or damage that causes an ongoing transfer of pain signals to the brain. Some examples of neuropathic pain include diabetic neuropathy (caused by long-standing high blood sugar levels), trigeminal neuralgia (a type of facial pain) and some types of cancer pain. Neuropathic pain doesn’t respond to common painkillers and often requires more complex treatments.
They conclude that ketamine initiated an avalanche of events that desensitised pain processing in the brain and spinal cord and interrupted the vicious circle of chronic pain.Ketamine: potent painkiller and antidepressant
Traditional painkillers such as paracetamol are not likely to help much with controlling challenging types of chronic pain. Stronger traditional painkillers, such as opioids, can be used temporarily, but they eventually lead to problems such as drug dependence. Antidepressants and anti-epileptic (anti-seizure) medications are often used to control chronic pain, but they also come with limitations and side effects. When all these fail to work, pain experts sometimes use more specialised treatments, including ketamine treatment.
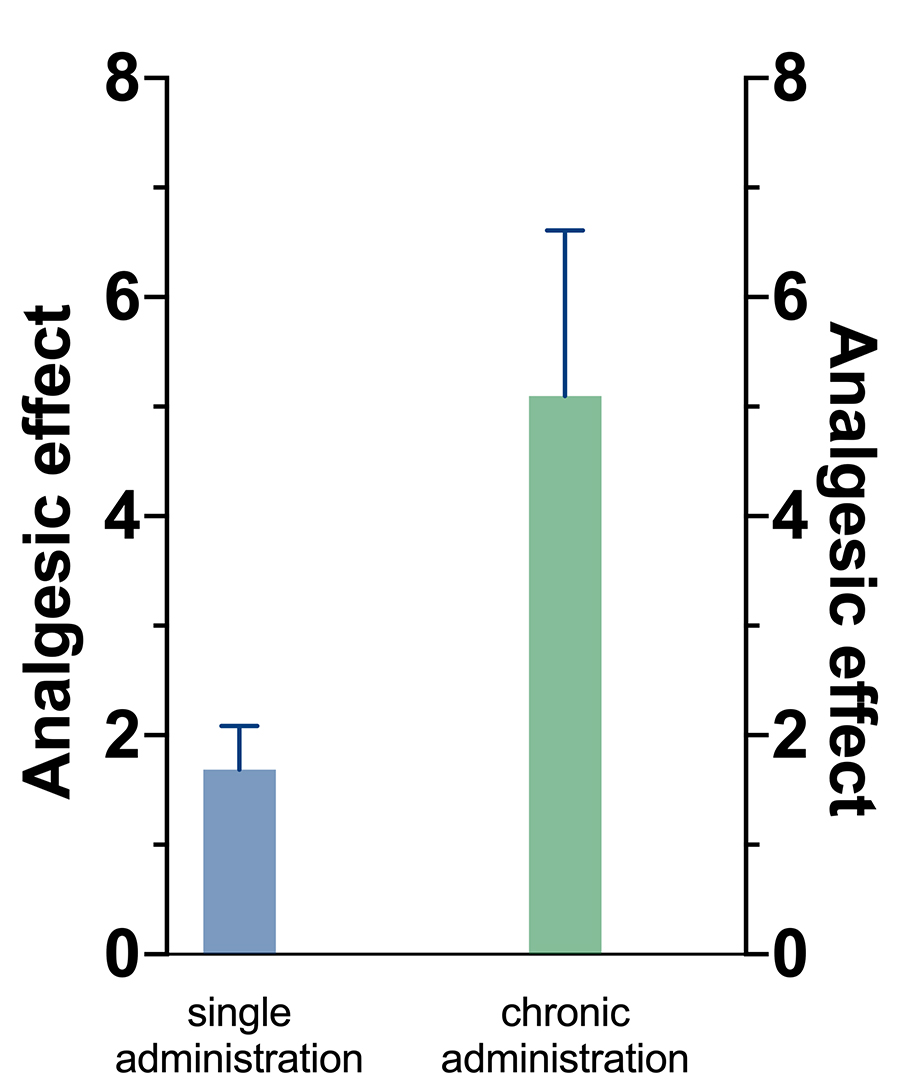
Ketamine is a drug that was initially used in anaesthesia, but since the 1990s it has also been used in a much lower dose as a treatment for acute and chronic pain. It is particularly helpful in the management of persistent chronic pain, especially since it can break the vicious circle of pain and sensitisation of the nervous system. Ketamine also works as an antidepressant and is often used by psychiatrists for treating patients with persistent depression.
Professor Albert Dahan and his team at the Leiden University Medical Center in the Netherlands have been studying this versatile drug for over 20 years. Through their long research work, they have managed to unlock some of its previously unknown mechanisms of action against pain, its psychedelic effects, but also new actions on the heart and breathing mechanism.
Managing pain with ketamine
To investigate ketamine’s potential in managing complicated cases of chronic pain, the team conducted a study involving 60 patients with CRPS-1. For the study, half of the participants were given a 100-hour intravenous infusion of low-dose ketamine, and the other half were given a placebo. The ketamine’s pain relief effect was evaluated by recording the participants’ pain scores (from 0–10) during a 12-week period. Analysing the data revealed that the pain scores of the patients who received ketamine were lower compared to those who had the placebo, with the lowest of all average scores recorded at the end of week 1 (ketamine 2.68 ± 0.51, placebo 5.45 ± 0.48). Towards the end of the 12-month period, there was no significant difference in the pain scores between the two groups.

The team’s next study of ketamine’s effects on managing CRPS-1 involved an analysis technique called PKPD modelling (a combination of pharmacokinetics and pharmacodynamics). This time, they took blood samples from the patients to measure ketamine levels in relation to pain levels. After analysing the data collected, the researchers were surprised to discover that ketamine’s pain relief effect lasted for 50 days after the termination of treatment. They conclude that ketamine must have initiated an avalanche of events that desensitised pain receptors in the brain and interrupted the vicious circle of chronic pain, an effect that persisted even when ketamine molecules were no longer there.
To better understand ketamine’s pain-relieving effects in the brain, Dahan’s team decided to look into the brain activity of healthy people. They designed a study involving 12 healthy volunteers who received either ketamine or a placebo. Their scores for pain and side effects were recorded, and towards the end of the treatment they underwent a functional Magnetic Resonance Imaging scan (fMRI) of their brain, a real-time investigation that reveals the activated areas of the brain. The changes observed in the scans and in relation to pain scores confirmed ketamine’s effect on the areas of the brain that are responsible for pain perception. It also appeared that ketamine changed the connections in the areas inside the brain that deal with self-regulating endogenous pain modulation, a type of pain relief that originates inside the brain and subdues external stimuli at the level of the spinal cord.

When it comes to managing neuropathic pain with ketamine, there is little knowledge based on studying humans. To obtain a better understanding of these actions, the researchers reviewed over 30 studies performed on animals. More specifically, they looked into ketamine’s effect on relieving allodynia, a sensitivity to stimuli that would normally not cause pain. The conclusion was that ketamine successfully relieved allodynia when given for a long time, an effect lasting several days after the treatment.
The results confirmed that ketamine can reverse opioid-induced respiratory depression.A mind-altering drug
In the above studies, the team also kept track of the psychedelic effects of the medication, including hallucinations and anxiety. The results suggested that the two functions are connected and most likely come from the same area in the brain. The findings initiated a discussion between researchers regarding ketamine’s psychedelic nature. It was suggested that the mechanism through which ketamine works is best described by the term ‘psychoplastic’ since it works by rewiring certain connections in the brain in a beneficial way, unleashing the healing properties of ketamine.
To further investigate its psychedelic properties, Dahan’s team conducted another study on healthy volunteers who were given ketamine infusions. The researchers measured changes in the perception of reality in correlation to the participants’ tolerance of pressure pain and the corresponding levels of ketamine in their blood. The results revealed a connection between ketamine’s pain-relieving effect and dissociation, the involuntary detachment from physical reality.
A further study of ketamine’s psychedelic properties again involved healthy volunteers who all received ketamine. Some also received nitric oxide, a messenger molecule that among other functions also regulates the pathway through which ketamine rewires the brain, while the rest were given a placebo. By analysing the participants’ symptoms, the team concluded that reduced nitric oxide levels amplify ketamine-induced psychedelic symptoms.
Actions on heart and breathing
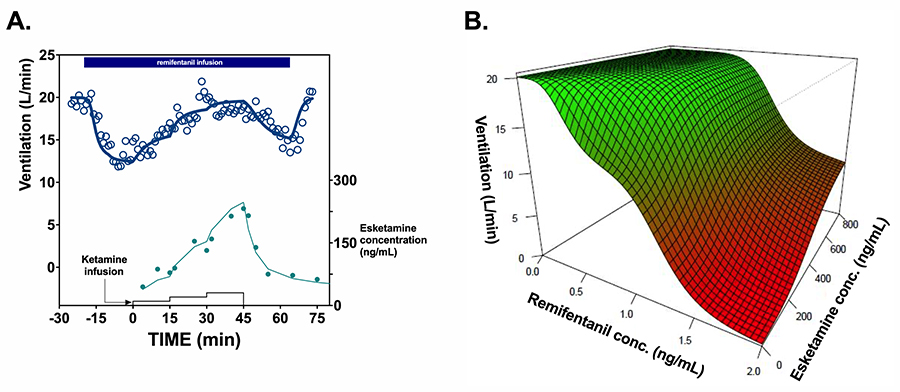
Ketamine’s effects on the human body are not restricted to brain-altering actions. Dahan and his team have also conducted experiments regarding its effects on respiratory and heart function. It is well known that opioids, a category of strong painkillers also called narcotics, can suppress breathing, sometimes putting patients’ lives in danger. To test ketamine’s potential for reversal of this side effect, the team tested 12 volunteers who were given ketamine or a placebo on top of an opioid called remifentanil. Measurements of vital signs, including breathing, and the levels of the medications in their blood circulation were taken and analysed. The results confirmed that ketamine can reverse opioid-induced respiratory depression.
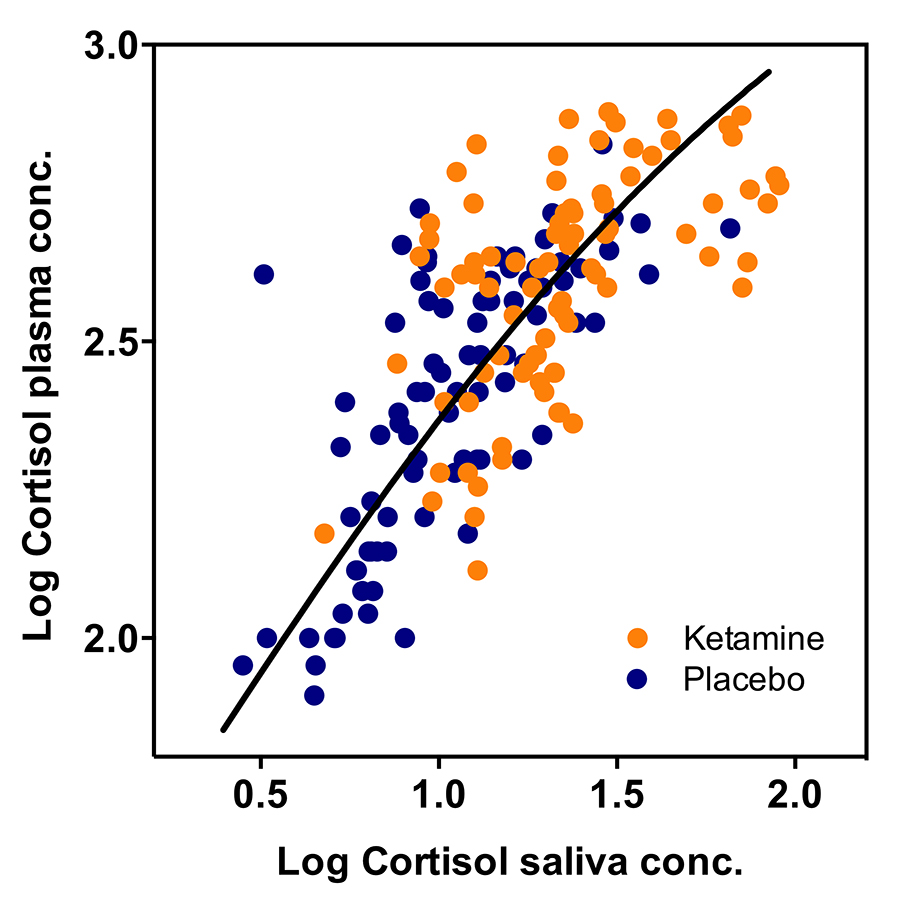
The team also tested ketamine’s effects on the heart’s potential to pump blood and the levels of stress caused by the medicine by designing two further studies. In one they measured the volume of blood pumped by the heart in relation to the blood levels of ketamine and nitric oxide, and in the other they recorded the levels of the stress hormone, cortisol. The results of their experiments revealed that ketamine increases both the heart’s pumping capacity and the levels of stress.
Ketamine is a versatile medication that has been long used as an anaesthetic and, over the past couple of decades, also as a potent treatment against difficult-to-manage types of chronic pain and depression. Besides further advancing the knowledge on its pain-relieving and brain-rewiring mechanisms, Dahan and his team have taken their studies further and looked into its seemingly unrelated actions, including its role in reversing opioid-induced respiratory depression and its effects on heart function and regulation of stress levels. These discoveries open a window for new research that can further establish the use of ketamine in treating mental disorders and also find its role in regulating critical physiological functions.

Personal Response
What do you think the potential future uses and applications of ketamine will be in treating mental illness but also respiratory and circulatory diseases?Many of the novel and recently discovered properties of ketamine are important and will be applied to treat millions of patients, such as those with treatment-resistant depression and chronic pain that is insensitive to traditional treatment. Furthermore, ketamine will reduce the need for opioids in the treatment of acute and chronic pain and simultaneously improve breathing during opioid therapy. We expect that ketamine’s indications will expand, for example towards treating post-traumatic stress disorder. Moreover, ketamine’s mechanism of action will lead to the discovery of novel compounds that can be used in depression or as reversal agents of opioid-induced respiratory depression.