Lateral flow test detects SARS-CoV-2 neutralising antibodies
To infect a host cell, virus particles—such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—use molecules on their outside surface to interact with cell surface receptors on host cells. Viruses must invade a host cell in order to replicate and build new virus particles.
Immune responses to viruses
Antibodies make up our immune system and defend our cells from pathogens by neutralising any harmful effects they have on the host cell, so they are no longer infectious or pathogenic.
There are two categories of antibodies against SARS-CoV-2: neutralising antibodies (NAbs, which make up a small fraction of antibodies), and non-neutralising virus binding antibodies (BAbs).
Specifically, NAbs block the interaction between the receptor-binding domain (RBD) of the spike protein of the SARS-CoV-2 virus and its receptor on the host cell, human angiotensin-converting enzyme 2 (ACE2). When NAbs bind to the ACE2 binding site on the RBD, the spike protein cannot fuse to the host membrane or invade the cell. Thus, NAbs eliminate the infectious virus particle before any infection can occur. In contrast, the majority of non-neutralising BAbs bind specifically to the pathogen but do not interfere with its infectivity. Their role is to flag the invading pathogen for other immune cells to destroy.
Testing NAb levels is vital for managing the spread of the coronavirus and to identify individuals who have to isolate.
Why is a NAb lateral flow test needed?
At the time of writing, there have been more than 120 million cases and 2.65 million deaths from COVID-19 globally. Governments across the world are rolling out vaccination programmes and biotechnology companies are mass producing vaccines. This needs to be achieved as quickly as possible, so that communities can obtain a protective level of neutralising antibodies, or ‘herd immunity’. This is important to kick start the economy and return to a semblance of normality after a year of battle against this novel coronavirus.
At present, there are many vaccines under development, with more than 30 in various stages of clinical trials. Almost all vaccines contain the virus’ spike protein, so can induce NAb activation to block virus entry into host cells. As governments attempt to control the spread of the virus, a rapid test, such as a lateral flow test, is needed if we are to break the cycle of lockdowns and restrictions. NAb levels are closely correlated with vaccine effectiveness and have been used as the primary laboratory measurement during vaccine development. Such tests that can continuously monitor NAb levels are required to determine the large-scale success and longitudinal efficacies of vaccination programmes, and to prevent the spread of further infections.
Achieving protection from SARS-CoV-2
Although multiple vaccines to the SARS-CoV-2 virus have been developed and approved, no single vaccine provides protection to 100% of its recipients. For example, the protection rate of Pfizer-BioNTech’s vaccine is 95%, while AstraZeneca’s protection rate is 70%. It is the development of a protective level of NAbs that promises some defence against infection or reinfection. By mass-measuring vaccinated recipients’ NAb levels, those who are not protected by the vaccines can be identified and revaccinated with a different vaccine or booster to achieve herd immunity.
There are still many unknowns when it comes to solving the puzzle of this novel coronavirus. For example, it has been predicted that antibody responses for the virus can be detected in a majority of infected individuals 10 – 15 days after the onset of symptoms and remain elevated after initial viral clearance. However, it is still not definitively known how long the biological antibody responses will be maintained, and if they can provide protection from reinfection. The duration of antibody responses required for communities to reach herd immunity is also not yet known. Levels of NAbs produced varies greatly between patients, and developed IgG antibodies (the main type of antibody) in patients with mild symptoms have a rapid half-life of approximately 36 days. Reinfection cases have also reported, baffling researchers as to the level of immunity that can be developed.
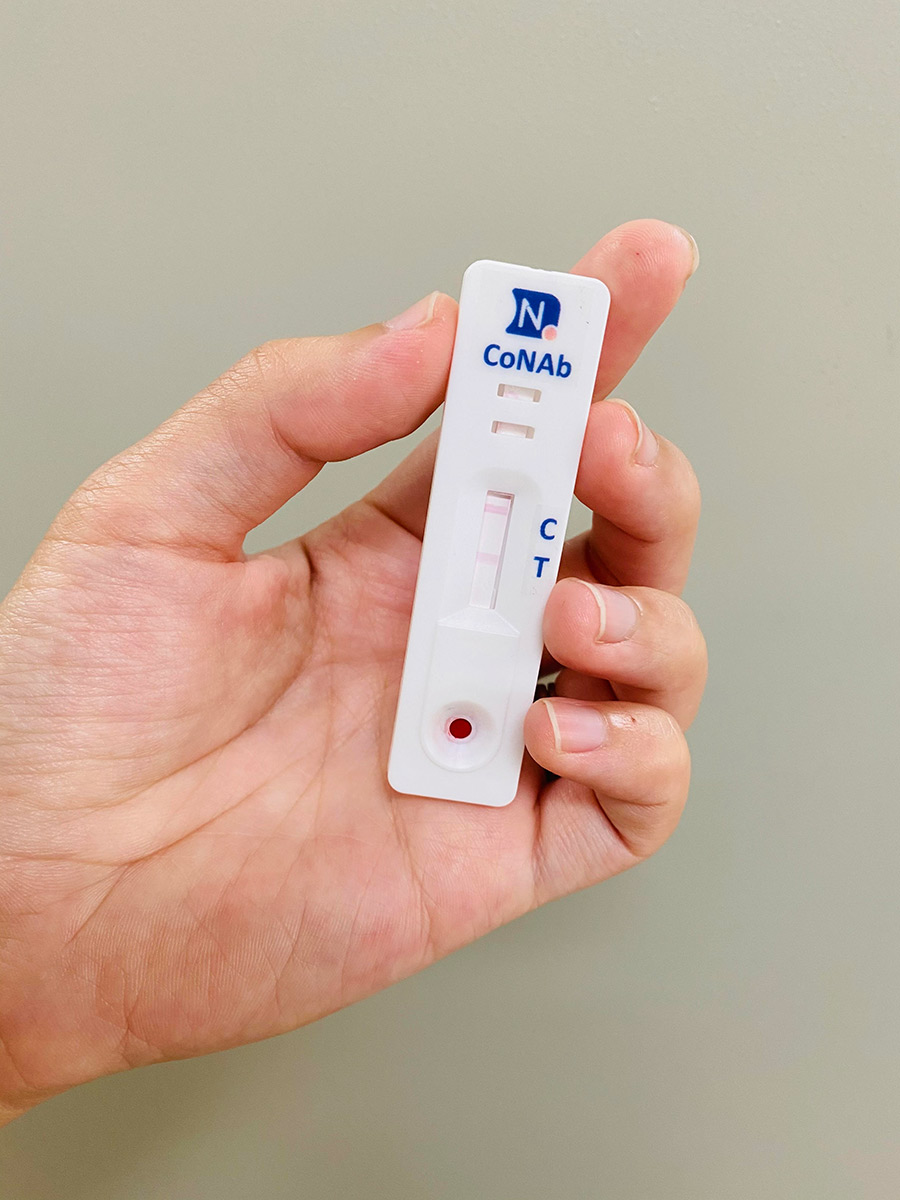
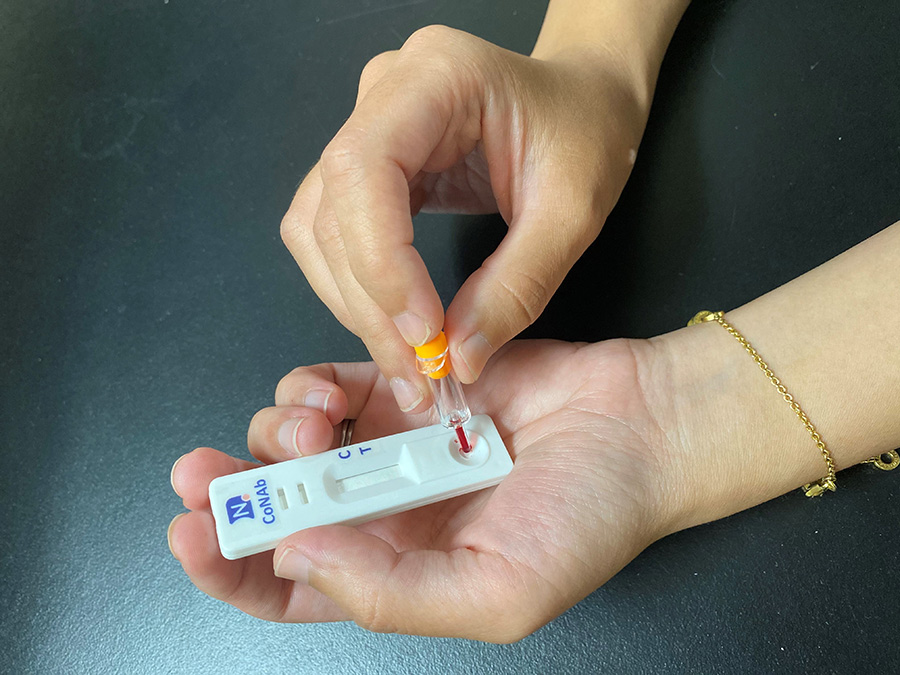
Testing for NAb levels is therefore essential to manage the spread of the disease and to identify individuals who have to isolate. A quick and easy method of measuring NAb levels can ensure regular testing within communities. This data could be used by government officials and policymakers to determine the timeline of lifting lockdown restrictions, to more accurately anticipate the course of the pandemic, and to better understand our immunology against this novel coronavirus, aiding the development of future strategies. It can also inform researchers about the role NAbs play in eliminating the virus – including the longevity of the protection they offer – and can protect communities from reinfection. In addition, the data can be used to monitor levels of protective immunity amongst vaccinated individuals.
CoNAb by Novodiax
Novodiax Inc. was founded in California in 2009, with a mission to advance immunoassays through innovation. Novodiax, under the guidance of lead researcher, Dr Jianfu Wang, has developed a rapid novel lateral flow device (LFD) to measure NAbs which are active against SARS-CoV-2, called CoNAb. LFDs are versatile, relatively inexpensive, and do not require laboratories with specialist equipment to provide rapid results. They also only take minutes to obtain results, compared to other methods, such as ELISA tests, which may take hours or even days. The Novodiax LFD works by detecting viral proteins (antigens) from a sample. If there are enough target antigens in the sample, a visual signal will be generated.
The rapid NAb test kit works by testing for the inhibition of the spike protein of SARS-CoV-2. Gold nanoparticle (GNP) is bound to the RBD of the spike protein while ACE2 (the target receptor on the host cell) is embedded onto a specialised nitrocellulose membrane on the test strip. Like all lateral flow tests, approximately 10µl of blood is taken from the patient and transferred onto the sample pad on the LFT device (Fig 1). 50 – 70µl of buffer is added to drive the blood sample through the device. Any NAbs present in the blood sample will neutralise or block the interaction between the RBD and ACE2. If NAbs are not present (e.g., the person being tested does not have immunity), then RBD and ACE2 will interact and the gold nanoparticles will be detected, producing a visual signal. However, if NAbs are present, then any GNP signal will be diminished, or will disappear.
Even though individuals are vaccinated, it should not be assumed that they have complete immunity against the virus.
At present, there are various methods to determine an individual’s NAb levels. Firstly, conventional virus neutralisation tests are conducted in a biosafety level three laboratory. These tests use live ACE2-expressing cells and SARS-CoV-2 or pseudovirus, and take 3 – 14 days for results. Although the gold standard for NAb measurement, this method is labour intensive and requires specialist laboratory working conditions. A simpler and faster enzyme-linked immunosorbent assay (ELISA) has also been developed. The ELISA mimics the ACE2-RBD (on the spike protein) interaction and detects the amount of SARS-CoV-2 NAbs in a few hours. However, the ELISA must be conducted in a specialist biosafety level two laboratory. In contrast, the CoNAb lateral flow test is very rapid, taking just 15 minutes to complete. It is also the most convenient, as it can either be performed by a professional staff member or by an individual at home.
A rapid and accurate test
The Novodiax team compared the efficacy and effectiveness of the CoNAb LFD against conventional methods such as ELISA and pseudo-virus neutralisation tests and obtained highly comparable results as indicated in the references cited. Blood plasma samples were obtained from a Bio-Bank company and an in-house collection: 50 were PCR positive for SARS-CoV-2, 30 were negative, and 80 were normal plasma obtained between 2014 and 2016 to establish negative baselines. There was very high agreement (91.14%) with RT-PCR, 72.92% sensitivity (the ability to correctly identify SARS-CoV-2 patients), and 99% specificity for SARS-CoV-2 (the ability to correctly identify positive cases).

In conclusion, herd immunity against SARS-CoV-2 is still far from a reality. Even though individuals are vaccinated, it should not be assumed that they have complete immunity against the virus. The measurement of NAb levels in individuals is needed to inform policymakers and scientists to confidently predict the epidemiology of the virus. The rapid and convenient LFD test developed by Novodiax can help determine the effectiveness of COVID-19 vaccine. It could also provide a basis for immunity passports for individuals who have developed SARS-CoV-2 neutralising antibody (rather than those who have just received the vaccine).
A broad collaborative effort is now required to up-scale and optimise this product, so it can be utilised widely across different communities.
Personal Response
How long will it take until the test is ready for general use?
<> Novodiax is currently preparing applications for U.S. Emergency Use Authorization, and for equivalent regulatory requirements in other countries. Meanwhile, we are ordering large quantities of lateral flow manufacturing materials. We anticipate that a product for general use will be released around June 2021.