Gestational diabetes: A novel detection strategy
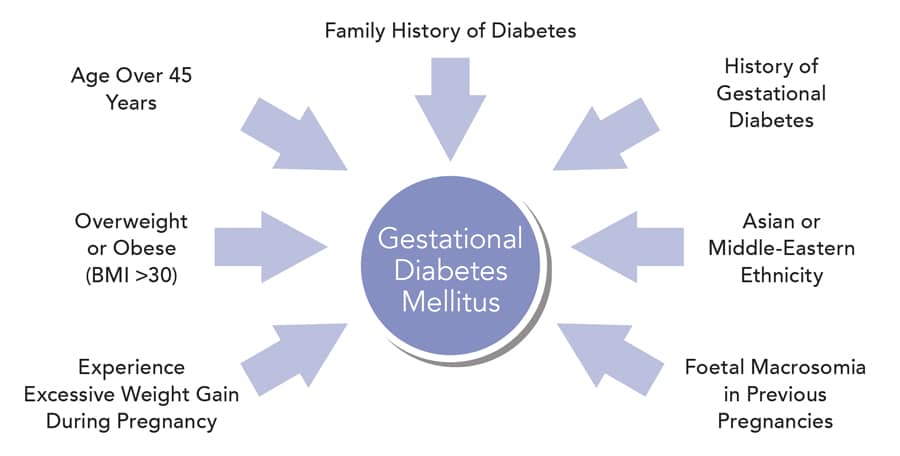
Gestational diabetes mellitus is a form of diabetes that may develop during pregnancy. The condition can arise at any time during pregnancy, however it most commonly occurs during the second and third trimesters, and resolves once the pregnancy ends. Gestational diabetes is caused by the diminishing action of insulin (insulin resistance) due to increasing hormone production by the placenta, and is associated with chronic inflammation. Women at risk of developing gestational diabetes are those who are older in age, overweight or obese (with a body mass index [BMI] greater than 30), experience excessive weight gain during pregnancy, or have a family history of diabetes. Other risk factors include previous gestational diabetes, Asian or Middle-Eastern ethnicity and foetal macrosomia (a large baby for gestational age) in previous pregnancies.

Notably, gestational diabetes is the leading cause of high blood glucose levels (hyperglycaemia) in pregnancy, the effects of which can lead to complications that affect both the mother and child. These complications include foetal macrosomia, preeclampsia, and polyhydramnios (too much amniotic fluid in the womb), all of which can result in premature labour and birth trauma. Babies born to mothers with gestational diabetes also have a higher lifetime risk of obesity and of developing diabetes. It is estimated that hyperglycaemia in pregnancy affects more than 16% of live births, with over 80% of these cases due to gestational diabetes. Also, pregnant women with hyperglycaemia are at higher risk of developing gestational diabetes in subsequent pregnancies and are estimated to be at seven times greater risk of type 2 diabetes development than women with normal pregnancies. Worryingly, mothers with gestational diabetes or with hyperglycaemia in pregnancy are also at high risk for causing other transgenerational effects for their offspring, such as a higher risk of hypertension and kidney disease.
The pre-screening blood test would eliminate the burden and cost of having to perform OGTTs in women at low risk of gestational diabetes.
The treatment of gestational diabetes reduces the risk of associated complications. However, the condition is most commonly diagnosed in late pregnancy, which leaves limited time for any intervention. Overt symptoms of hyperglycaemia during pregnancy are rare and can be easily confused with normal pregnancy symptoms. Consequently, gestational diabetes is universally diagnosed using an oral glucose tolerance test (OGTT) performed between the 24th and 28th week of pregnancy – a procedure that is time-consuming and arduous for both the clinic and the attending pregnant women. Furthermore, the negative impact of maternal hyperglycaemia on the foetus may be underway before conventional late pregnancy gestational diabetes screening is performed. Thus, any intervention given after a diagnosis late in pregnancy may have limited effect. Importantly, many pregnant women experience nausea and discomfort when performing the OGTT, therefore it is of interest to limit the number of women who have to undergo the procedure to only encompass those at higher risk of gestational diabetes. An accurate blood test capable of identifying women at low or high risk for gestational diabetes already in the first trimester of pregnancy would have the potential to reduce the need for universal testing in all pregnant women, decrease costs, and improve outcomes through prevention or treatment.

To meet this challenge, Dr Jonatan Dereke, an assistant researcher and member of the Diabetes Research Laboratory team at the Department of Clinical Sciences Lund, Lund University, Sweden, and his colleagues, Dr Magnus Hillman, Dr Mona Landin-Olsson, Dr Charlotta Nilsson, Dr Helena Strevens, and Birgitte Ekholm, are endeavouring to develop a simple, non-fasting, pre-screening blood test that can be used before the OGTT to identify women at low or high risk of gestational diabetes. With the assistance of funding from the Gorthon Foundation and Skåne University Hospital Funding and Donations, Dr Dereke and his team are currently investigating several proteins present in the blood circulation during early pregnancy that could act as biomarkers, allowing the identification of women at increased or decreased risk of developing gestational diabetes.
One of the key areas of Dr Dereke’s research is the investigation of pregnancy-associated plasma protein-A2 (PAPP-A2), a protease cleaving insulin-like growth factor-binding protein. PAPP-A2 is most abundantly expressed in the placenta, where it is found to regulate foetal-placental growth and placental development. In healthy pregnant women, PAPP-A2 increases as the pregnancy develops, with levels becoming stable during the third trimester. To determine the connection between PAPP-A2 and gestational diabetes, Dr Dereke and his team conducted a case-control study in which circulating PAPP-A2 was analysed in two groups of women in early pregnancy – one group consisting of pregnant women who had been diagnosed with gestational diabetes following an OGTT performed as part of a general screening in southern Sweden, and the other group consisting of pregnant women without diabetes. The women in both groups were matched for age and BMI, to minimise any bias these risk factors may have had on the results. This provided a unique and heterogeneous patient population increasing the validity of the results, as there was no selection bias at recruitment.


The study revealed that women diagnosed with gestational diabetes in early pregnancy have increased circulating PAPP-A2 levels compared with pregnant women of a similar gestational age not diagnosed with diabetes, independent of BMI, C-peptide (a by-product of insulin production from the pancreas) or adiponectin (an adipocyte-derived protein that enhances insulin sensitivity and is inversely associated with obesity and diabetes risk). The study also demonstrated that if PAPP-A2 is employed as a pre-OGTT screening biomarker, one third of women in early pregnancy, who are at low risk of developing gestation diabetes, will no longer need to receive an initial OGTT. This outcome would eliminate the burden and cost of having to perform OGTTs in women at low risk of gestational diabetes, while minimising the number of women with gestational diabetes missed. Notably, this is the first study to address the topic of pre-screening biomarkers for gestational diabetes. PAPP-A2 might also be able to predict gestational diabetes development in late pregnancy; however, further prospective studies are required to fully evaluate this association.
Notably, this is the first study
to address the topic of pre-screening biomarkers for gestational diabetes.
A number of other novel biomarkers in early pregnancy may also serve as potential predictors of gestational diabetes. For example, Dr Dereke and his team have determined that plasma levels of soluble tumour necrosis factor-like weak inducer of apoptosis (sTWEAK) – a protein implicated in numerous cellular processes – are significantly decreased in women with early pregnancy gestational diabetes compared to levels in pregnant women without diabetes. Additionally, Dr Dereke and his colleagues have conducted a study of relaxin-2, an important gestational hormone involved in shaping the endometrium in early pregnancy. Higher levels of relaxin-2 were observed at 12 weeks of pregnancy complicated by gestational diabetes than in pregnant women without diabetes.
Taken collectively, the findings from these studies suggest that, in addition to PAPP-A2, there are several potential biomarker candidates for early pregnancy gestational diabetes that could be detected using a simple blood test. Going forwards, Dr Dereke and his team are planning further investigations to confirm their results.

Personal Response
Please could you provide further details on the next steps for your research and highlight what will be the key focus?
<> Our next steps are to recruit a larger group of pregnant women and measure potential biomarkers for gestational diabetes that are present throughout the whole duration of the pregnancy. This prospective cohort study will give us more insight into the biological events contributing to gestational diabetes development as pregnancy progresses. It will also help us to find even better biomarkers of disease development. We have also started studying the connections between gestational diabetes and the effect on children’s growth development and risk for becoming overweight and obese later in life. Taken together, these studies will help us better understand the underlying pathogenesis for gestational diabetes and its consequences for future generations.