Understanding neonatal brain injury proteinopathy: Implications for adult-onset neurodegenerative disease
Neonatal encephalopathy is damage to the brain caused by the disruption of its blood supply before and during childbirth and other reasons such as prematurity and maternal-foetal infection. It can often lead to death. Survivors can have long-term cognitive, emotional, and behavioural effects. Lee J Martin, Professor of Anaesthesiology and Critical Care Medicine and Pathology at Johns Hopkins University, USA, has investigated the different aspects of neonatal brain injury for many years. He believes that the molecular events involved could help explain the mechanisms behind later-onset diseases including Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia, and traumatic brain injury with important implications for the development of innovative treatments targeting proteinopathy.
Hypoxic-ischaemic encephalopathy (HIE) is a form of brain damage in infants caused by the compromised blood and oxygen supply to the brain. HIE affects between one to three infants in every 1,000 live term births in the USA and Western Europe. Worldwide, almost one million infants die every year from HIE. Its consequences vary from moderate long-term cognitive impairment to more severe complications such as cerebral palsy and death. The only approved treatment for HIE is hypothermia, the use of mild low temperatures to cool the infant’s body or head about 4°C cooler than usual for 72 hours. Then, the baby is rewarmed to ambient room temperature. Unfortunately, this method is used on only some babies depending on the time after the insult, the anticipated severity of brain injury, and hospital resources. In some instances, therapeutic hypothermia, as the method is named, can prevent death but can only make future symptoms milder. It does not entirely prevent damage to the brain and has uncertain effects on abnormal brain electrical activity associated with HIE. Thus, the morbidity can still be high and can be life-long.
There is an urgent unmet need to improve the outcome of infants with HIE. Professor Lee J Martin and his lab group at Johns Hopkins University School of Medicine, USA, studies the neuropathology and mechanisms behind neonatal HIE brain damage and believe that by unlocking them further we can achieve a better understanding of the nervous system and its postnatal development. The team aim to discover how neurodegenerative diseases throughout the life-long spectrum (conditions that gradually damage and destroy parts of the nervous system) emerge at cell, molecule, and system-wide levels, and help develop innovative treatments to prevent future brain damage in infants and children that might be leveraged on other major diseases occurring in adults, such as Alzheimer’s disease, Parkinson’s disease, and traumatic brain injury.
HIE and brain connectivity
The nervous system is an exquisite network made up of billions of connections between nerve cells (neurones) and different regions of the brain responsible for processing information, emotions, learning, memories, decision-making, and motor skills. The neurone-to-neurone connections occur essentially at cellular kisses called synapses between the end of a neurone’s axon, identified as the presynaptic terminal, and part of another neurones’ architecture such as the cell body, dendrite, dendrite spine (a protuberance on a dendrite), or even axon. The connectome is the map of these connections and the arrangement or organisation of how different parts of the brain are connected and communicate using neurotransmitters such as glutamate, dopamine, and acetylcholine.

Many other non-neuronal cells in the brain, called glial cells with spectacular names like astrocytes and oligodendroglia, are also involved in this communication. Astrocytes function in neurotransmitter, electrolyte, and water uptake. Oligodendrocytes function in support and ensheathment of axons from many different neurones. Recent studies on the connectome, seen by state-of-the-art brain imaging methods such a magnetic resonance imaging (MRI) and its many scanning variations, have highlighted the important role it plays in many human brain injuries and diseases, including neonatal HIE, traumatic brain injury, and Alzheimer’s disease. More specifically, after a hypoxic-ischaemic insult, the different neonatal brain areas can sustain different amounts of damage, depending on the levels of their vulnerability. The cellular and molecular basis for this selective vulnerability is not understood and is likely to have therapeutic relevance. For example, certain neurotransmitter binding sites called receptors might be involved. Moreover, different brain regions have different levels of metabolic activity driven by how active or supercharged the mitochondria (the cellular powerhouses) are in constituent groups of neurones.
The formation of prion-like proteins and their likely transfer to other areas of the brain via the connectome could lead to progressive neurodegeneration and even Alzheimer’s.Mitochondria need oxygen to function properly in producing the energy currency of a cell called ATP, and when brain blood flow or oxygen content is reduced, mitochondria become abnormal and can be Trojan horses that ultimately cause cellular damage. There might also be differences in the propensity for different neurones to engage cell death pathways along what is known as the cell-death continuum that consists of a spectrum of different pathological forms. These varying levels of vulnerability, as well as the connectivity between different areas of the brain, have been recently mapped in a piglet model of hypoxia-ischaemia by Martin’s laboratory.
Experimental HIE model
The study involved four groups of piglets (with six piglets in each group): those that received hypoxic-ischaemia (HI) without treatment (HI piglets); those that received HI and treatment with whole body hypothermia (HI-HT piglets) administered by a water blanket with circulating temperature-regulated water; piglets that had no HI injury and no cooling treatment so they were always at normal normothermic (NT) body temperature (sham-NT piglets); and those that had no HI injury but were nevertheless treated with hypothermia (sham-HT piglets) to see if the body cooling by 4°C (38°C to 34°C) causes harm. The sham groups are called controls.
Before HI and sham procedures, the piglets were neurosurgically equipped with EEG recording devices for continuous monitoring of brain activity for seizures. The piglets were euthanised at specifically matched times after the HI and sham procedures and their brains were prepared so that they could be studied in detail at the microscope. This preparation involved cutting the brains into thin sections and using special dyes and antibodies to proteins to identify neurones so that possible relationships could be made between the HT treatment, neuronal damage, and presence or absence of abnormal electrical activity called seizures.
Normal and damaged neurones in different areas of the brain were counted. For HI piglets, the counts revealed varying damage severity in the anterior and posterior motor, somatosensory, and frontal cortices, areas that are neighbouring in both humans and pigs and that are responsible for decision-making, problem-solving, motor function, speech, personality, and environmental sensations. Interestingly, the HT treatment strongly protected against some of the HI neuropathology but not so much against the abnormal seizure activity. Moreover, the HI insult caused an abnormality in the neurones’ nucleus where the cells’ DNA is housed and where this genetic code is transcribed into RNA. The nucleus lost a protein called Rbfox3. Rbfox3 has critical functions in processing of RNA. HT did not fully protect against this important pathology.
These findings further support the idea that therapeutic HT is not fully protective and that human children that survive neonatal HIE and HT treatment can have epilepsy and impairments in executive, visuospatial, and emotional-social functions, as well as language skills because of the diverse vulnerability found in different areas of the brain and the way these areas are connected to each other. These neurological alterations might have their basis in molecular abnormalities involving how RNA is processed, edited, distributed, and translated to protein within vulnerable neurones of the connectome.
Proteinopathy might have a role in neonatal HIE
Proteinopathy refers to irregularly structured or modified proteins that lead to the disruption of certain cell functions that can consequently affect the health and normal activity of tissues and organs. For example, pathological proteins can interfere with pores or channels in the cells’ cell membrane, mitochondria, or nucleus. They can clog a key garbage disposal-like organelle called the proteasome that degrades proteins. These protein irregularities can be in the form of abnormal folding or bending and the formation of assemblies of similar proteins into aggregates or structures called oligomers and fibrils. Theses aggregates can be relatively dissolvable in the brains’ water content (which is about 80% of the brain) and are called soluble, or they can be relatively insoluble clumps in water. Some harmful modifications of proteins can be brought about by attack from reactive oxygen species (free radicals) released by abnormal mitochondria. Some abnormal proteins can be highly toxic to cells. They are called toxic conformer proteins.
Drug-induced proteasome activation is possible and therefore the proteasome might become a future therapeutic target for treating human neurodegenerative diseases.Proteinopathy and toxic conformers occur in the nervous system of people with Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and diabetes. After an HI injury, important cell proteins such as tubulin and pericentriolar material-1 (PCM1) become severely damaged by oxidative modification. Tubulin is a protein essential for structural cell support, transport of molecules and cell division, while PCM-1 is a protein necessary for the normal function and stability of neurones. Martin’s experimental piglet HIE model was used to study the fate of key proteins thought to be critical in some adult-onset neurodegenerative diseases.
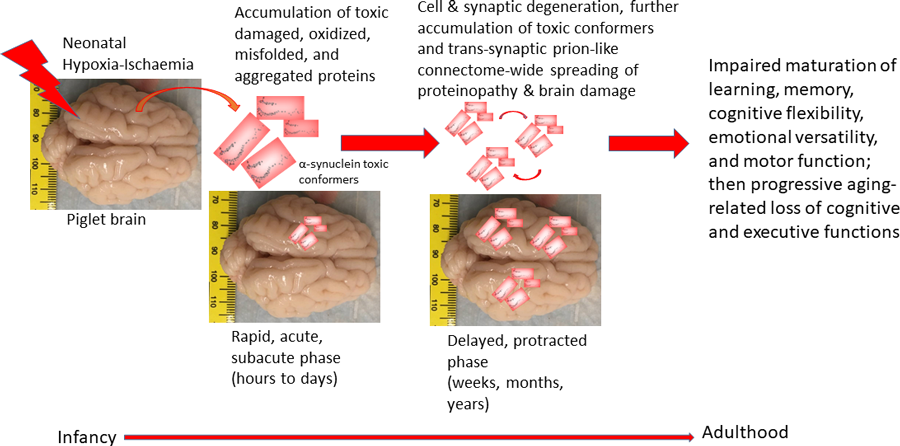
What they found was astonishing. They observed that proteinopathy occurs very quickly in the brain. Irregular and aberrant proteins accumulated relatively rapidly in different areas of the brain because of the induced HI injury. One protein called α-synuclein, an abundant synaptic protein, is strongly implicated in Parkinson’s disease and in dementia. Another protein called superoxide dismutase-1, an abundant antioxidant protein, is implicated in the pathogenesis of amyotrophic lateral sclerosis (ALS). These observations contrast with the dogma that it takes years for these abnormal proteins to accumulate as believed in Alzheimer’s disease, Parkinson’s diseases, and ALS. More specifically, HI induced the formation of prion-like proteins (structural abnormal proteins that resemble those that cause transmissible neurodegenerative diseases such as ‘mad cow disease’) in piglet neonatal brain (see Figure 1). Martin and his colleagues suspect that these damaged proteins can be transferred between cells and different areas of the neonatal brain via the connectome pathways by essentially seeding and spreading through specific brain networks during childhood and into adulthood.
Neuronal cell death and the cell-death continuum
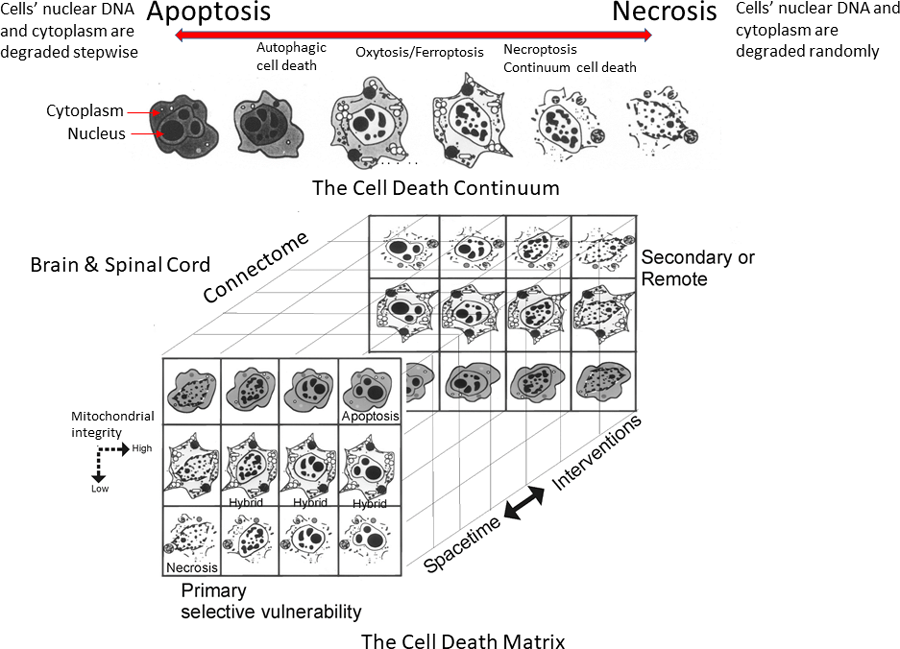
The nervous system consists of two broad classifications of cells: neurones, the main neural cells responsible for synaptic vesicle release mechanisms and the transmission of neurone-to-neurone signals, and glia (astrocytes, oligodendrocytes, and microglia), the cells that provide neurones with structural and metabolic support and function in synaptic signalling modulation and innate immunity. Acquired injury to the newborn brain as in HIE causes complex pathology in part because of the ongoing normal cell development, cell death, and maturation. Neurones and glial cells in the newborn brain can be lost normally in a process called programmed cell death (PCD). Generally, this process of PCD is structurally and biochemically very ordered and named apoptosis (see Figure 2, top). PCD is necessary for the development and formation of human tissues and the removal of DNA-damaged and cancerous cells.
Apoptosis involves a fascinating family of enzymes called caspases that become activated to systematically destroy, in a regulated process, cellular constituents (including the DNA and cytoplasm), ultimately leading to large-scale, yet precise, digestion or fragmentation of the cells’ DNA into microscopic packets (blebs) that can be eaten or phagocytosed by nearby cells. This event is related to the brain connectome because it serves to match populations of projection neurones to the size of the target to which they project. In a way, the brain is much like a complex neural matrix comprised of nodes of cells (see Figure 2, bottom). It is unclear how neonatal HIE alters this brain region-to-region matching and connectivity consolidation process (the matrix assembly) and whether the brain cooling and rewarming done for therapeutic HT alters deleteriously this normal process in any way. Martin’s group has shown that brain cooling and rewarming itself, in the absence of HI injury, can indeed cause some neuronal and glial damage and apoptosis in piglet brain. Thus, the clinical use of therapeutic HT needs scrutiny in the framework of neuropathology in postmortem brains from infants when its implementation was unsuccessful.
In contrast to apoptosis, necrosis is the type of cell death where a cell has been abruptly and severely damaged through trauma, stroke, or disease resulting in rapid mitochondrial impairment and loss of cellular ATP and membrane energisation, ion and water fluxes, swelling, rupture of the cell membrane and cell contents being released, a process that can often cause inflammation in nearby tissues (see Figure 2, top). Some of the triggers for necrotic neuronal cell death in the brain involve too much synaptic release or too little glial uptake of the excitatory neurotransmitter glutamate, leading to excessive activation of potent stimulatory glutamate receptors and a toxic process called excitotoxicity.
In Martin’s neonatal piglet HIE model, the necrotic form of neuronal cell death predominates, at least within the first seven days after the HI insult, in contrast to the relatively low level of classic apoptotic cell death. Interestingly, Martin’s group found in neonatal brain injury, originally using excitotoxicity, that there are also alternative types of cell death in between necrosis and apoptosis. These are hybrids or syncretic forms referred to as the apoptosis-necrosis continuum, where the cell both swells up and gets dismantled in an organised way into small packages before its components are broken down by the nearby cells. Finally, autophagy is a mechanism by which cells degrade their own components and recycle their proteins and other useful nutrients. Sometimes this process can transition to autophagic cell death, a type of cell death linked to neurodegenerative diseases.
These different types of cell death are believed to be involved in the mechanism of neurodegeneration and nerve cell death. The degeneration and death of neural cells after neonatal HI brain injury can be acute, subacute, delayed, and protracted in patterns that are governed by the neural matrix assembly of the brain, forming a three-dimentional cell death matrix over time (see Figure 2, bottom). This pattern could be explained by the model of the apoptosis-necrosis continuum. Martin’s lab group is also now studying the contribution of the different forms of cell death in postmortem brain samples from human infants that died from HIE. These tragic autopsy cases will be scientifically invaluable to understand the cell death mechanisms occurring in the human HIE brain and how the neurodegeneration in animal models compares to the human HIE (this is critical to show if the experimental systems are even relevant to the human condition and if we need to change the experimental modelling), if toxic conformer prion-like proteins are formed (see Figure 1), and how therapeutic HT and rewarming influence the human infant brain injury.
The proteasome and its potential importance to HIE
The current standard of care for babies with HIE is insufficient. Therapeutic add-ons to HT are needed desperately. Adjuvant treatments could be pharmaceutical drugs administered intravascularly or even by nasal spray. However, brain molecular targets for drugs need to be identified experimentally and interrogated for proof-of-principle and therapeutic relevance. Moreover, if new drugs are developed, they need to be tested for their dissolvability, target engagement, and therapeutic efficacy in translationally relevant experimental models. Martin and his colleagues believe that the cellular proteasome is one such target that could be critical for neonatal HIE novel therapeutics.
Proteasomes are complex molecular structures in the shape of a barrel with two open ends, inside the cells’ cytoplasm and nucleus that degrade proteins into smaller peptides. They break down some proteins to quickly shorten their lifespans (for example, some proteins stick around for only about one hour). Proteasomes also degrade senescent proteins and damaged and abnormally structured proteins such as the prion-like proteins formed in the neonatal brain after HI injury. Recent studies in newborn pigs have revealed exciting information on the concentration and function of proteasomes in different areas of their brain. Importantly, Martin’s group has also shown recently that intravascular administration of certain medications to piglets can increase proteasome activity in the brain. Some of the medications have been used on patients for other proposes and thus have a long record for safety. These findings suggest that drug-induced proteasome activation is possible and made the researchers hopeful that the proteasome could perhaps become a future therapeutic target for treating human infant HIE and adult neurodegenerative diseases.
Understanding neurodegeneration
These four pathological aspects of neonatal brain injury – connectomics, proteinopathy, cell death, and the proteasome – are likely to be interconnected. A further understanding of their role in neonatal HIE could open new research paths – all four can potentially play a future role as therapeutic targets for treating adult neurodegenerative diseases. The formation of prion-like proteins in the neonatal brain after HI injury and their likely transfer to other areas of the brain via the connectome might be the root cause of the known neurological, cognitive, and behavioural consequences through childhood to adulthood, but potentially also results in a persistent and progressive neurodegeneration that can later in life lead to diseases such as Alzheimer’s and Parkinson’s diseases (see Figure 1). Martin’s future studies aim to explore this hypothesis further, with the aim of homing in on therapeutics for neurological disease that target and activate the brain proteasome in diseases along the human lifelong continuum.
Personal Response
You developed an animal model to aid the study of neurodegenerative disease. Can you tell us a bit more about this?
The newborn piglet has many distinct advantages over other experimental animal systems, such as neonatal rats and mice, to uncover the mysteries human pathobiology and to translate these findings to the clinical bedside for therapeutic needs. For example, the piglet can be treated and monitored in ways very similar to the approaches used in hospital neonatal intensive care units (see Figure 3).
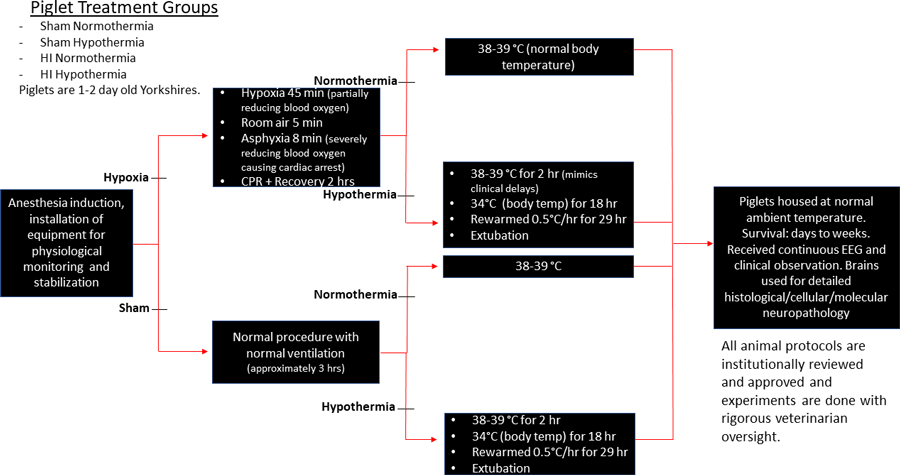
This involves careful intensive monitoring of body temperature, respiration and lung functioning, heart functioning and blood pressure, blood chemistry such a pH, oxygen content, electrolytes, and glucose, and brain electrical activity by electroencephalography (EEG). When hypothermia is implemented, the piglets need to be anaesthetised during the entire treatment, so they need to have round-the-clock observation and monitoring by highly skilled team members. Piglets can receive intravascular drugs to treat physiological perturbations.
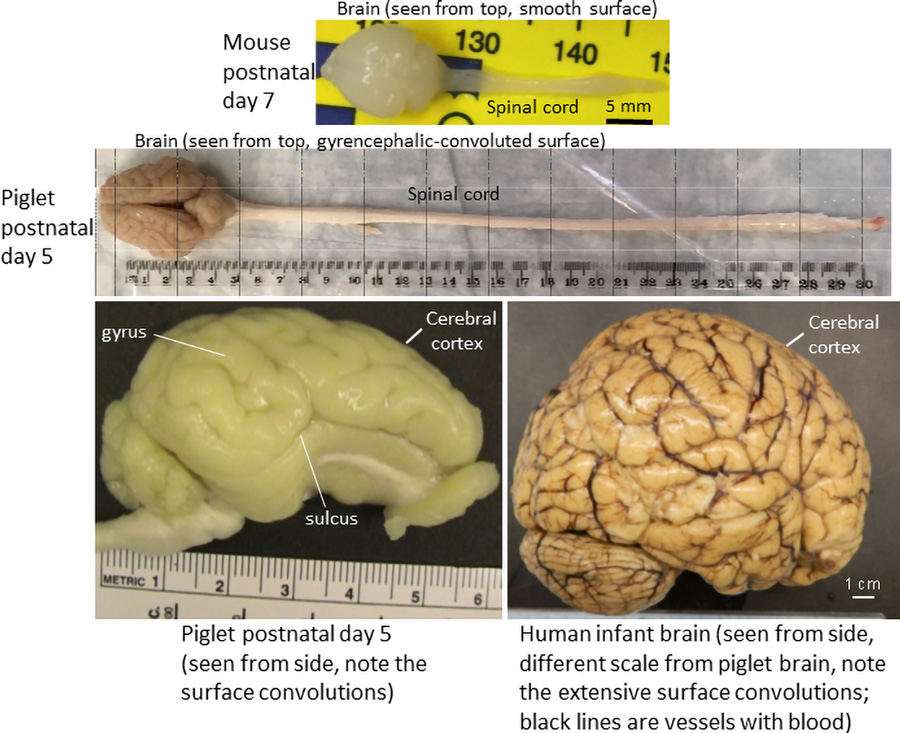
An additional major advantage is that the piglet brain neuroanatomy (see Figure 4) and its neurocytology are very similar to the human brain, including a gyrencephalic (convoluted) cerebral cortex and the presence of wondrous giant neurones in the primary motor cortex called Betz cells. Lastly, piglet neurones respond to injury, engage cell repair, and die more similarly to human neurones than do mouse neurones. Newborn non-human primate brains are the ultimate best match to the human brain, but the ethical and emotional burdens of using neonatal monkeys are very heavy and the costs are nearly prohibitive. The piglet is a reasonable middle of the evolutionary road choice between rodents and monkeys for experimentally modelling human neonatal brain damage. All protocols used on piglets are institutionally approved to minimise animal suffering and ensure humane treatment, all experiments are rigorously monitored by institutional veterinary doctors, and all efforts, including statistical predictions, are used to minimise the number of animals used for the experiments.