Crystal engineering for solid-state molecular recognition
Molecular recognition is a process through which molecules interact to form non-covalently bonded assemblies, often comprising multiple components and exhibiting complex structures. The binding of these complementary molecules arises from weak interactions, including hydrogen bonds, metal coordination, π···π and π···metal interactions, van der Waals forces and electrostatic attraction. Molecular recognition occurs naturally in several biological processes, including antigene-antibody interactions and DNA-protein binding, and it is at the core of the mechanism of action of specific classes of drugs, like antibiotics. Synthetic systems exhibiting molecular recognition have also been created, and they have been put forward as promising materials for the selective manipulation of small molecules in a variety of technological applications.

Solid-state frameworks with a highly porous structure have received particular attention as flexible “smart hosts” for small- to medium-size molecules, in view of their potential ability to bind and transport molecules and bio-antibody models with high efficiency and selectivity. Host frameworks can be designed by exploiting weak interactions between their building blocks, to form porous network structures with cavities capable of accommodating guest molecules. However, controlling the growth of the crystal network, and, especially, the size and structural properties of the host cavities can be challenging. Frequently, unexpected transformations occur in the crystal structure during the incorporation of guest molecules and cavity mismatch can occur in consequence to the formation of interpenetrating structures.
Fluorine is a unique substituent that dramatically changes the intramolecular electron withdrawing nature and intermolecular interactions of aromatic materials.
To overcome the limitations of current materials growth processes, Professor Hori has been pioneering the synthesis and use of non-porous materials with high adsorption selectivity and efficiency, which are prepared through self-assembly of chemically functionalised monomers. In particular, she has been able to show that the fluorination of organic ligands can be used as a simple and powerful approach to prepare host materials with the ability to bind reversibly or irreversibly wide classes of molecular species, and to provide new promising solid-state media for molecular recognition.

Coordination complexes as building blocks
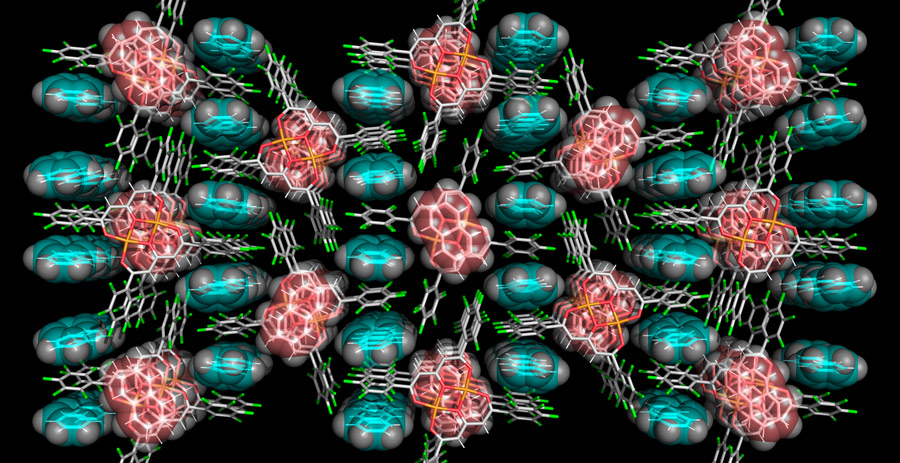
Host crystalline structures can be created through the self-assembly of discrete molecular species. The self-assembly process is spontaneous and is regulated by the size, shape and chemical properties of these building blocks. Transition metal coordination compounds composed of a metal cation surrounded by an organic ligand with extended aromatic bonds are good examples of self-assembling building blocks. These compounds exhibit a variety of structures and properties, which depend on the nature of the cation and of the coordinated ligand. The assembly of these monomers to form perfect crystals, however, is not controlled simply by the nature of the cation itself and by the external conditions under which the self-assembly process is carried out, like the temperature and type of solvent used during the crystallization.
Furthermore, the crystallization of coordination compounds typically only yields uniform crystals with a very regular structure and high structural rigidity, which makes them unsuitable for molecular recognition. Polymorphic materials, which can exist in different crystal structures, and pseudo-polymorphic materials, which adopt different crystal structures depending on the presence of water or other solvents, can provide suitable alternative routes for the development of synthetic systems for molecular recognition.
Control of crystal growth through fluorination
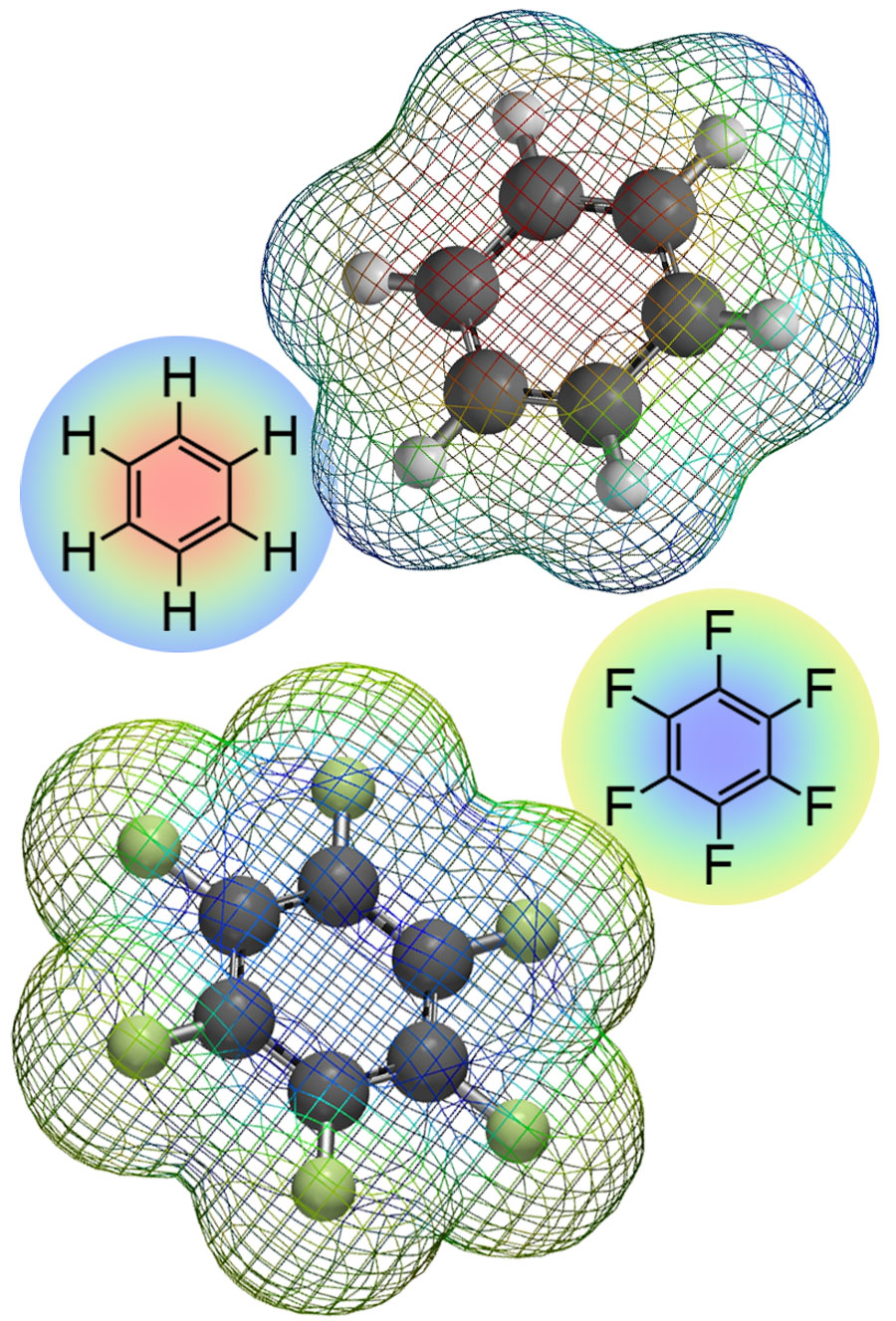
To favour the formation of pseudo-polymorphic crystals with enhanced host features, Professor Hori and her collaborators have developed a simple chemical approach, in which the molecular flexibility, electrostatic potential and local electron density of the monomers are modified through the addition of fluorine atoms in their molecular structure. Fluorine has a large intra-molecular electron withdrawing nature, which dramatically affects the intermolecular interactions between aromatic groups of ligands without significantly changing their physical properties and molecular structures. The perfluorinated aromatic rings show positive quadrupole moments (e.g. hexafluorobenzene is +3.2×10-39 C m2) to give completely opposite and different molecular recognition events. Solid-state samples grown from fully fluorinated monomers were found to exhibit much higher flexibility than their unfluorinated counterparts. This flexibility can be explained in terms of changes in the electrostatic interactions between the cations in the coordination complexes (which are enhanced by the presence of the electron withdrawing fluorine atoms), along with anion···π, CF···H and arene-perfluroarene (π···π-hole) interactions. Repulsive F···F interactions also play an important role. Altogether, these interactions are responsible for the existence of multiple states with different crystal structures, which is a crucial feature of systems exhibiting guest recognition properties.
Molecular recognition in fluorinated compounds
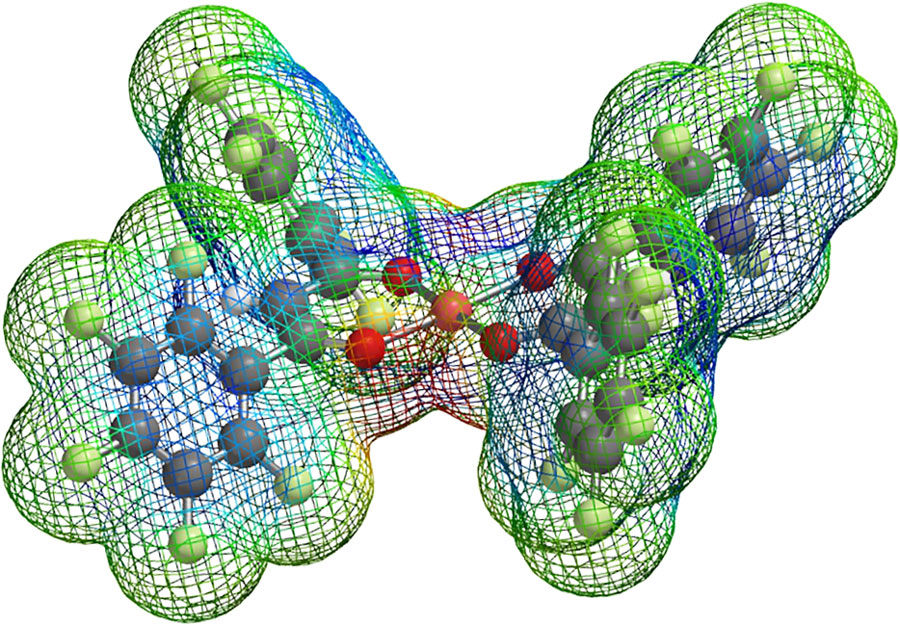
The research team have synthesised several flexible host frameworks of fluorinated mono-nuclear and di-nuclear coordination complexes of various transition metal cations (including Cu2+, Fe3+, Co2+, Ni2+, Pd2+ and Pt2+) with very specific molecular recognition properties. These frameworks show two types of guest recognition events: the selective binding of CO2 and the metal ion-selective adsorption of small gas molecules, such as benzene, N2, O2, H2, NO and CH4. Crucially, host structures obtained from the corresponding non-fluorinated coordination complexes show no tendency to guest encapsulation.
These interactions are responsible for the existence of multiple states with different crystal structures, which is a crucial feature of systems exhibiting guest recognition properties.
Quadrupolar host-guest interactions
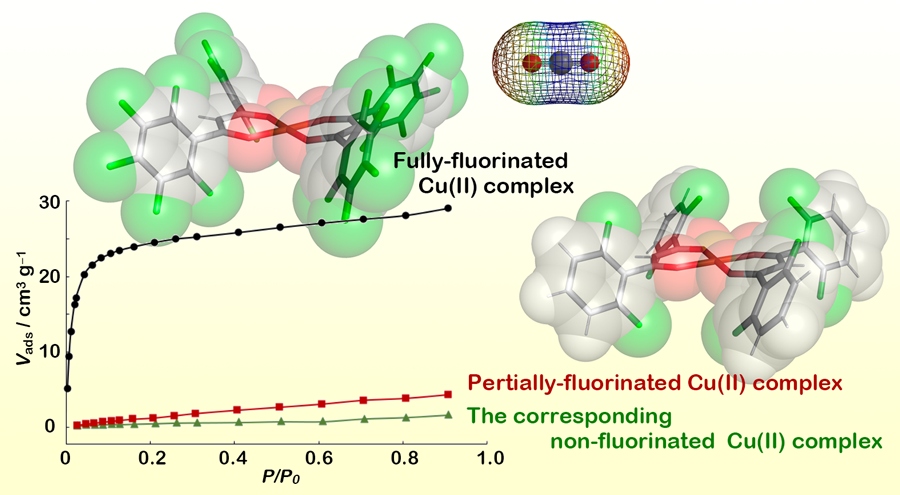
Professor Hori has also studied in detail the structural and electronic properties of hosts obtained from fluorinated complexes, to devise a model for the origin of the enhanced molecular recognition properties of these systems. She used x-ray crystallography measurements to understand what features in the local structure of the adsorption site are responsible for promoting the selective binding of specific guest molecules. For instance, in the case of fully fluorinated pentafluorophenyl ligands with Cu2+, she demonstrated that guest aromatic molecules (e.g. benzene derivatives) arrange themselves in the most suitable position, within the flexible cavities of the host, to establish direct π···cation and arene-perfluoroarene interactions. In this specific case, the planar nature of the ligand allows access to a free axial coordination site, which acts as a binding site for the guest molecule.
Furthermore, the ability of the cation to act as a coordination site is enhanced by the presence of fluorine atoms on the ligand, which promote the displacement of electron density away from Cu2+, effectively strengthening electrostatic π···cation interactions. The fluorine atoms also induce on a ligand a net quadrupole moment, promoting attractive interactions with guest molecules of opposite quadrupole moment. This explains the remarkable ability of the systems prepared by Prof Hori’s team to act as efficient hosts for non-polar and negative quadrupolar molecules like CO2 (-1.4×10-39 C m2) and benzene derivatives (e.g. benzene is -2.9×10-39 C m2).
Personal Response
Fluorination appears to be a very simple and general approach to obtain new materials with enhanced molecular recognition properties. How did you become interested and develop this approach, and what technological fields do you think will benefit most from your work?
<> The research of molecular recognition has moved from relatively strong interactions such as polarity and hydrogen bonding to weaker intermolecular forces. Since carbon dioxide and aromatic hydrocarbons, which are associated with environmental problems, are non-polar molecules, and both happen to have negative quadrupole moments, we noticed that the design of molecules with positive quadrupole moments using fluorine atoms is necessary for their recognition and separation. The technological fields of our research will benefit the purification and storage of molecules for chemical products and the separation of environmental pollutions.