More than a just a simple powerhouse: Structure and function of mitochondria
Everything we do requires energy. Much of that energy is generated by mitochondria: small, membrane-bound structures, or organelles, that are present in almost every cell in the human body (as well as in the cells of almost every other form of life). Some cells, such as those in the liver, muscle etc, contain many more mitochondria than other cells, such as blood cells. Often called the “powerhouses” of the cell, mitochondria take energy from food in the form of metabolites and convert it into a molecule called ATP, the energy currency that the cells in the body can use. As a byproduct of ATP production, mitochondria produce substances called reactive oxygen species (unstable oxygen-containing molecules).
While energy production is the most well-known function of mitochondria, it is certainly not their only role. Mitochondria are also involved in other crucial processes, such as cell signalling and homeostasis – maintaining stable internal conditions of the cells that impacts the bodily functions. In addition, mitochondria are known to play an important role in the complex processes of ageing, which involve damage by the mitochondria-generated reactive oxygen species over time and alterations of cell signalling and homeostasis supported by mitochondria.

The classic depiction of mitochondria is as an oval or sausage-shaped structure, filled with an irregularly-folded membrane. However, some studies indicate that this image is misleading, and that mitochondria exist in a range of shapes and sizes. In more recent decades, research has elucidated how mitochondria interchange between various shapes and sizes and the significance of these shape changes in various physiological processes and diseases.
Mitochondria are diverse in shape and structure
Even within a single cell, mitochondria can be heterogeneous; that is, they vary in both structure and function. Further, mitochondria are not only heterogeneous at the level of the whole organelle. Recent research suggests that, within a single mitochondrion, there are multiple different regions that are responsible for very different functions.
Dr Mitra’s work clearly suggests that regulation of mitochondrial dynamics is strongly integrated with regulation of the cell cycle.
As with other important pieces of biological apparatus, in mitochondria structure and function are likely to be inextricably linked. The different levels of mitochondrial heterogeneity and their implications are the research focus of Dr Kasturi Mitra at the University of Alabama. Together with graduate student Brian Spurlock, the team aims to understand the relationship between structure and function in mitochondria, both in health and disease.
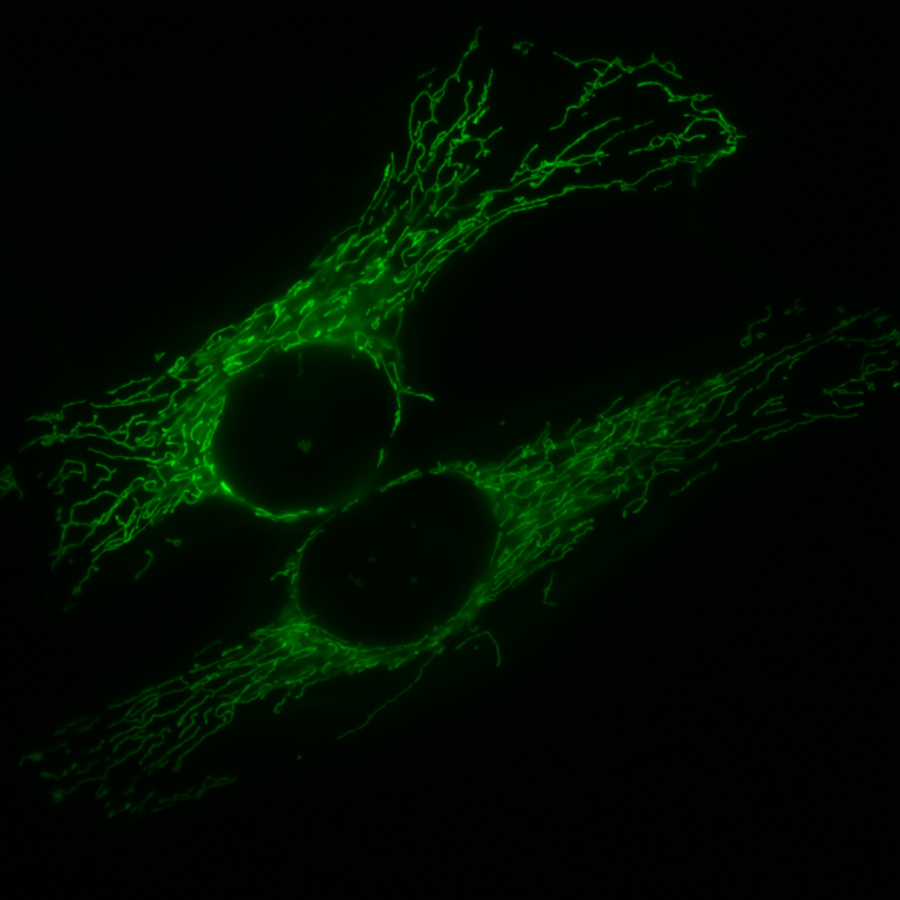
The structure of any individual mitochondrion, at any given time, depends on both the type of cell in which the mitochondrion resides, and the physiological state of that cell. The necessary variations in mitochondrial shape and structure, however, are created primarily by two opposing processes: fission and fusion.
Fission-fusion dynamics and shape change
Mitochondrial fission (splitting) and fusion (joining), collectively known as mitochondrial dynamics, are part of the complex of systems that keeps a cell in balance. During fission, one large mitochondrion splits to form smaller mitochondria. Conversely, during fusion, two mitochondria join together to form one large organelle, or to exchange their contents.
It is becoming increasingly clear that the fission and fusion dynamics of mitochondria serve to protect the cell. Fusion might allow damaged and undamaged mitochondria to join together, “diluting” the damage; on the other hand, fission could allow the organelle to dispose of a damaged part, which can then be broken down by the cell. In this way, fission and fusion act as “quality control” processes for mitochondria.
Control of mitochondrial dynamics is an intricate process. The balance of fusion and fission is maintained by specific molecules within the cell, particularly certain enzymes and other regulatory proteins. Interaction of mitochondria with other organelles and structures within the cell can also impact mitochondrial fission and fusion.

Mitochondrial dynamics and the cell cycle
Mitochondrial dynamics appear to be influenced by the cell cycle: the process in which a cell grows and divides to form daughter cells. According to Dr Mitra, mitochondrial shape change is fastest in cells that are proliferating, i.e. growing and dividing to increase in number. In these cells, the cell cycle swiftly repeats over a short space of time. The progression of the cell cycle is regulated by a family of proteins called cyclins, which activate various enzymes at different stages of the cycle. Cyclins also regulate key mitochondrial functions, including energy release and fission-fusion dynamics.
Dr Mitra and her team have successfully identified changes in mitochondrial shape associated with the cell cycle in cells taken from different species of mammal. Along with data gathered by other researchers, Dr Mitra’s work clearly suggests that regulation of mitochondrial dynamics is strongly integrated with regulation of the cell cycle.
Dr Mitra and her colleagues next aimed to understand the impact of mitochondrial shape change during the cell cycle. There is evidence that the amount of ATP produced by mitochondria varies according to the different energy demands of each stage of the cell cycle. Dr Mitra’s research revealed that ATP produced by mitochondria actively affect specific parts of the cell cycle. When mitochondrial ATP is depleted, the cell cycle may slow or stall. Her lab demonstrated that such slowing down of cell cycle is due to direct mitochondrial regulation of the cyclin called Cyclin E that is very easily destroyed by cellular protein degradation machinery. As a mechanism, they have proposed that energetically active mitochondria recruits Cyclin E on the mitochondrial surface to prevent its destruction and is released to support cell proliferation. Importantly, they showed that the master regulator of mitochondrial fission mediates the mitochondrial regulation of Cyclin E and thus the cell cycle.

By actively and directly participating in each stage of the cell cycle, mitochondria can influence the path a cell takes: will it become quiescent (or dormant), continue to proliferate, or differentiate (i.e. become specialised)? Research is ongoing to define exactly how this happens. Dr Mitra is particularly interested in discovering how the energy-production capabilities of mitochondria, as regulated by mitochondrial fission and fusion processes, contribute to determining the fate of a cell.
Mitochondrial dynamics in stem cells
Stem cells are cells which have not yet become specialised, or differentiated, but have the potential to divide into specific cell types. Dr Mitra’s findings, supported by the results of other researchers, suggest that cell cycle-related changes in the fission-fusion balance have particular effects on stem cells.
By actively and directly participating in each stage of the cell cycle, mitochondria can influence the path a cell takes.
Adult stem cells are mostly maintained in a quiescent state; that is, they are not actively growing or dividing. The quiescent cells are called into action when necessary during the lifespan of an organism. Keeping stem cells in the state is important in preventing them from becoming “exhausted,” or no longer able to produce the necessary specialised cells. It appears that mitochondrial fusion is vital in holding adult stem cells in their quiescent condition. When fusion is inhibited, fission allows the stem cells to begin the process of division and specialisation.
Mitochondrial dynamics are an essential part of the cell cycle, and therefore of survival. This applies to both “normal” cells and stem cells. Unfortunately, as with all biological processes, these systems can occasionally go wrong.
Mitochondria, cancer and ageing
Cancer arises when cells grow and divide in an uncontrolled manner. Dr Mitra’s research suggests that this out-of-control proliferation could be maintained by excessive mitochondrial fission in connection to their aberrant cell cycle. In recent work, Dr Mitra, Brian Spurlock and colleagues developed a new microscopy-based method, which they named mito-SinCe2, for quantifying the heterogeneity in mitochondria. Using this method, the team identified three distinct sub-populations of cancer cells, each with characteristic states of mitochondrial structure-function relationship. One of these three sub-populations has greater ability to maintain specific stem cell properties in the cells that can initiate and maintain tumours. This sub-population of cells have reduced mitochondrial fission and enhanced production of mitochondrial reactive oxygen species when maintained in cell cycle quiescence. Further experimentation led the researchers to suggest that these specific quiescent tumour-initiating cells when activated undergoes massive mitochondrial fission to likely support proliferation of the cancer cells in an uncontrolled fashion.
Imbalance in mitochondrial fission or fusion impacts lifespan of various model organisms, like worms, flies, mice and even in humans. Dr Mitra believes that their discoveries at the cellular level possibly contributes to age-related loss of cell proliferation. Disruptions in mitochondrial dynamics might affect cells in numerous ways, including by reducing their ability to respond to nutrients and by making the cellular genome unstable and prone to mutations. These faults can lead to age-related conditions, including cancer, diabetes and cardiovascular disease.
The work of Dr Mitra and her colleagues has advanced understanding of mitochondrial dynamics, while also opening up exciting new potential avenues of research. Through these discoveries, it has become clear that mitochondrial structure is deeply and inextricably linked with function, and that this relationship impacts cells at every stage of the cell cycle, from proliferation through to differentiation and ageing. Greater knowledge of mitochondrial dynamics could also lead to improvements in health, through shedding light on the processes behind the growth and spread of cancer.
Personal Response
Besides cancer, do we know of any other diseases in which mitochondrial dynamics might play an important role?
<> Various diseases, like diabetes, musculo-skeletal diseases, neurodegenerative diseases, metabolic syndrome, ageing, etc.